| AI in Clinical Medicine, ISSN 0000-0000 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, AI Clin Med and Elmer Press Inc |
| Journal website https://aicm.elmerpub.com |
Review
Volume 1, 2025, e5
Generative Artificial Intelligence as a Catalyst for Effective Cancer Treatments
Ted Rogers School of Information Technology Management, Toronto Metropolitan University, Toronto, ON M5B 2K3, Canada
Manuscript submitted July 25, 2025, accepted August 15, 2025, published online August 28, 2025
Short title: GenAI as a Catalyst for Effective Cancer Treatments
doi: https://doi.org/10.14740/aicm5
- Abstract
- Introduction
- AI in Oncology
- Fundamentally, Cancer Treatment Is a Control Problem
- Why GenAI Is a Potential Catalyst for Effective Cancer Treatments?
- Discussion
- Conclusions
- References
| Abstract | ▴Top |
Current standard of cancer care is supported by a continuously expanding set of therapeutic options becoming available to cancer patients such as KRAS inhibitors, third-generation tyrosine kinase inhibitors (e.g., osimertinib), new immunotherapy drugs (e.g., durvalumab), as well as adaptive and combination therapies involving chemotherapy, radiotherapy, immunotherapy and targeted therapy. Despite these advances, therapeutic resistance persists as an inevitable challenge to cancer cure. While combination therapy is a plausible strategy to thwart therapeutic resistance, further research is needed on rationalizing drug combinations. On the other hand, adaptive therapy is emerging as a sound strategy to counter the evolving nature of cancer and thwart or delay the onset of therapeutic resistance. Indeed, cancer is a nonlinear time-varying dynamical system whose treatment can be viewed as a problem of steering the disease to a desired end-state of cure or stable management based on monitored treatment response. The success of this strategy depends on accurate and reliable estimations/predictions of disease state and tumor growth dynamics. Therein lies the potential of generative artificial intelligence (GenAI) and its underlying large language models (LLMs) to leverage the accumulating big clinical, radiomic and molecular data about cancer patients and their treatments to power its learning and predictive capabilities towards assisting treatment decision-making. This perspective starts with examples of current therapeutic advances and a succinct overview of persisting challenges to the development of effective cancer treatments, followed by a broad survey of artificial intelligence (AI) applications in oncology and their varying degrees of clinical readiness. Given this context, cancer treatment is framed as a problem of controlling a nonlinear time-varying dynamical system, where data-driven GenAI learning and predictive capabilities would be instrumental in resolving the challenges of disease monitoring and controllability. The perspective shares insights about potential pathways to GenAI-assisted improvement of cancer treatments and discusses key challenges to its deployment in real-world clinical settings, including data curation, clinical validation, LLM hallucinations and ethical concerns. Ultimately, advancing noninvasive tracking of treatment response dynamics and curating corresponding big LLM training datasets are essential to the potential of GenAI as a catalyst for effective cancer treatments.
Keywords: Generative artificial intelligence; Cancer treatment; Clinical decision support systems; Personalized treatment; Rapid learning decision-support systems; Adaptive cancer therapy; Large language models; Dynamic treatment scheduling; Precision oncology; Cancer therapeutic resistance
| Introduction | ▴Top |
Significant advances have been achieved in expanding cancer armamentarium with newly approved targeted therapy, immunotherapy and combination therapy drugs. For instance, combining trastuzumab with chemotherapy is now standard of care for metastatic and early stage human epidermal growth factor receptor 2 (HER2)+ breast cancer [1]. The recent Food and Drug Administration (FDA) approval of KRAS inhibitors sotorasib and adagrasib for KRAS-G12C mutated non-small cell lung cancer (NSCLC) patients opens avenues for combination therapies involving chemotherapy and immunotherapy [2]. Likewise, trastuzumab deruxtecan (T-DXd), which was recently approved for any HER2-expressing solid tumor [3, 4], is under evaluation in combination with durvalumab for advanced or metastatic NSCLC [5]. Although combination therapy is a mainstay of standard of care and an essential strategy to thwart the emergence of resistance, often, however, it is not clear which patient would respond to which drug and what combinations of drugs and doses will improve patient outcomes and maintain their effectiveness throughout the course of treatments. These challenges are rooted in the adaptive complexity of cancer [6-10] driven by its genetic [11-16], immunological [17-21] and eco-evolutionary [22-26] dimensions. The evolving nature of cancer, the lack of effective biomarkers, the limited number of available patients compared to the number of possible drug combinations, the cumulative toxicity of drug combinations, and the lack of effective techniques for continuous and accurate monitoring of treatment response are critical barriers to the development of effective cancer treatments that can decisively thwart therapeutic resistance and improve overall survival and quality of life (QoL). On the other hand, the success of generative artificial intelligence (GenAI) and its underlying large language models (LLMs) and expanding application space [27-30] compel the application of their learning capabilities to the accumulating molecular, clinical and radiomic data about cancer patients towards decisive improvement of cancer treatments [31-34]. In oncology, leveraging GenAI’s potential towards more effective clinical-decision making is garnishing increased research efforts [35-39], which are aligned with pre-GenAI initiated research [40-47] regarding issues that are inherent to deep-learning-based artificial intelligence (AI) systems, including data, ethics, transparency, explainability, accountability, privacy, ethics, and deployment in real-world clinical settings. In addition, the use of GenAI requires the mitigation of LLM hallucinations and the availability of large datasets and adequate computing infrastructure to carry out training and fine-tuning of LLMs. Fundamentally, however, the application of GenAI to assist treatment decision-making rests on the assumptions that cancer state dynamics can be estimated from treatment response observations, and that disease state can, at will, be steered using clinically feasible treatments, vis-a-vis toxicity, to a desired curative or managed end-state. Satisfying these assumptions in practice faces challenges caused by cancer complexities (e.g., evolving spatiotemporal heterogeneity of tumors, cancer immunoediting, and phenotypic plasticity) and by the scarcity of effective treatment response biomarkers and their degrees of faithful sampling of disease state.
The proposed perspective frames cancer treatment as an adaptive control problem and argues that GenAI data-driven learning and predictive capabilities hold the potential for addressing the challenges of estimating/predicting disease state and tumor growth dynamics, which would make it critically instrumental to the realization and clinical adoption of adaptive cancer therapy. The article starts with an overview of AI applications in oncology, followed by an elaboration on the nature of cancer treatment as a problem of controlling nonlinear dynamical systems. The subsequent section argues the utility of GenAI as a potential catalyst for effective cancer treatments and addresses elements that are critical to its clinical realization. Key real-world clinical issues are highlighted in the discussion section emphasizing that affording them adequate attention would be essential to facilitating the exploitation of LLM learning and predictive capabilities towards improved cancer treatments, and in particular the thwarting of therapeutic resistance.
| AI in Oncology | ▴Top |
The advent of AI in oncology is driven by its learning and inferencing capabilities which are harnessed from the increasingly large clinical, molecular and radiomic cancer datasets to assist in cancer diagnosis, prognosis and treatment decision-making. The growing and dynamic knowledge base being available about cancer and its treatments, combined with the expanding space of cancer therapeutics and the steadily growing number of diagnosis, prognosis and risk stratification biomarkers being validated, are providing the impetus for an ever-growing exploration of AI and machine learning (ML) applications in oncology [35-56]. These research efforts are matched with a growing list of AI/ML software as medical devices (SaMDs) approved by the FDA for oncology [57]. By the end of 2024, there were 1,016 AI/ML devices that met FDA pre-market requirements, spanning 17 categories of medical applications. More than 76% of approved AI/ML devices are dedicated to radiology while cardiovascular and neurology have 10% and 4% shares, respectively, leaving a combined slice of about 10% for the remaining categories, which include gastroenterology-urology (1.3%) and pathology (about 0.3%). Three notable examples of approved AI/ML SaMDs for cancer diagnosis are the Transpara™ system for mammography, the GI Genius™ for colonoscopy and Paige Prostate Alpha for whole slide images (WSIs). Transpara™ uses a deep learning (DL) model to provide a breast cancer likelihood score from 1 (low risk) to 10 (high risk) [58], while GI Genius™ relies on convolutional neural networks (CNNs) to support lesion detection during colonoscopy for colorectal cancer (CRC) screening or surveillance [59, 60]. On the other hand, Paige Prostate Alpha exploits CNNs and recurrent neural networks (RNNs) to detect prostate cancer and points to areas where the detection is made with high probability [61]. It is also worth noting that, when supported with Paige Prostate Alpha, pathologists’ classifications of low-grade prostate cancer are more likely to be correct [61]. Aside from cancer diagnosis applications where AI use is steadily progressing, AI-driven risk stratification and prognostication are attracting increased research attention [43, 45-47, 62-69]. A similar research trend is underway for the use of AI and GenAI in treatment selection and recommendations [38, 42, 70-75]. However, the path to clinical translation of AI-assisted treatment decision-making approaches has been particularly challenging, with a noticeable lack of prospective studies - save for a few exceptions, such as the clinical integration undertaken by McIntosh et al on ML-driven planning of radiotherapy for prostate cancer patients [42]. Among the most vexing challenges to clinical translation is the “black box” nature of AI systems, which leads to an opacity that exacerbates the challenges associated with an already complex and multifactorial clinical decision-making process [76-78]. Adding to the “black box” concern, there is a myriad of hurdles to the undertaking of prospective studies that are rooted in the complexities inherent to the dynamic nature of data-driven AI systems and their performance drift in time and across patient populations, as well as in the possibility that traditional designs of randomized control trials (RCTs) may be ill-suited to evaluate such complexities [79]. Other barriers are born out of other concerns such as the “dataset shift” and the need for its governance [80], non-universal generalizability of AI benefits and their dependence on the clinical context of their deployment, and the disconnect between AI evaluation metrics and their clinical applicability [81]. Cultural and regulatory challenges are equally significant and include the reticence of clinicians towards the use of AI in clinical decision-making [82], and the complexity of navigation through the regulatory system, including institutional research boards (IRBs). Nevertheless, there is a mounting research interest in the application of GenAI in oncology [32, 36-39, 83-85], as well as an increasing number of interventional and observational studies that are either planned or ongoing (e.g., NCT04675138, NCT05681949, NCT06986564, NCT07045207), where measures of concordance between treatment recommendations of clinicians versus that of GenAI represent recurrent primary outcomes for a significant portion of these studies. The focus on treatment recommendations is presumed to be an attempt to leverage the medical knowledge embedded in off-the-shelf LLMs such as ChatGPT [36]. Evaluations of these models yielded mixed results in terms of performances and limitations with respect to their potential use in real-world clinical settings [35, 85-88], highlighting the limits of general-purpose LLMs. These limitations may be overcome through the use of dedicated LLMs, whose trainings are confined to the oncology knowledge base, with the expectation that such customization would yield more robust LLM learning and predictive capabilities to assist in clinical decision-making for oncology.
| Fundamentally, Cancer Treatment Is a Control Problem | ▴Top |
The different cancer therapeutic modalities in use, whether systemic or targeted, are developed with the presumption that cure and disease management are realizable through actions on one or more of the driver dimensions of cancer (i.e., genetic, immunological and eco-evolutionary). Significant cancer treatment advances have been achieved whereby combination and adaptive therapies are emerging as critical components of effective cancer treatments. On the other hand, there are persisting barriers to the development of cancer therapies that can deliver long-term disease management or cure, including the hurdles facing the search for effective drug combinations, the cumulative toxicity of combination therapy, and the challenges of predicting tumor growth evolutionary dynamics to support adaptive therapy. Fundamentally, cancer treatment is a control problem [89], where the objective is to manage or cure the disease by adapting drug combinations and dosage based on real-time feedback provided by repeated monitoring of treatment response while limiting toxicity. The coupled working of genetic dysregulation of cell signaling and metabolic pathways, immune system defenses, and eco-evolutionary pressures, shape tumor growth dynamics under therapeutic intervention and make it highly unlikely for therapeutic strategies to achieve long-term disease management without an integrated accounting of these drivers of cancer adaptive complexity. This is strongly illustrated by the persistence of unmet needs of cancer patients despite the availability of an impressive number of cancer drugs, often developed to address one lever of cancer control (e.g., tyrosine kinase inhibitors, PARP inhibitors, immune checkpoint inhibitors, etc.). While further advances in deciphering the biology of cancer and its response to treatments are needed, such advances should be recast into computational models and supported by mathematical abstractions that enable an integrated consideration of the various dimensions underlying treatment response dynamics. One such abstraction is the view of tumors as time-varying nonlinear dynamical systems, where drug doses and treatment response are their input and output, respectively, while tumor clonal composition represents disease state [89]. Cancer treatment can then be formulated as the problem of controlling a nonlinear dynamical system [89-92], where the timing and doses of administrated drugs are adapted based on real-time treatment response monitoring with the objective of steering the disease to a desirable end-state. This typically involves treatment adaptation schemes that are informed by mathematical and computational models of the system under control, i.e., the tumor, and are fed by real-time disease state feedback [90-92]. Mathematical models of tumors embody conceptual and descriptive perspectives rooted in assumptions inspired by insights into possible determinants of cancer dynamics such as evolutionary competition [93]. Among these, models that are used to support adaptive control capture the behavior of cancer under treatment in the form of disease state trajectories, which can be steered through a series of controls towards a desired end-state. While this formulation provides an actionable mathematical representation of cancer’s nonlinear dynamics, it has challenges regarding the questions of system observability and controllability [94-98]. The system, i.e., the tumor in this case, would be fully observable if all disease states (i.e., clonal frequencies) can be uniquely determined from observations of treatment response. Partial observability would apply if some, but not all disease states can be determined from treatment responses. While theoretical results on the analysis of nonlinear system observability [99-101] may be used to assess the observability of tumor dynamics, it is not clear whether such assessment would yield clinically useful insight, given the reductionist assumptions that would have to be made about tumor growth dynamics to undertake the analysis. A more practical approach to the assertion of system observability would be to identify biomarker panels of treatment response that are established to be strongly correlated with disease state. An example of concrete steps to support this approach would be to accelerate undergoing efforts in establishing the utility of circulating tumor DNA (ctDNA) and developing optimal protocols of its use (e.g., optimal timepoints of liquid biopsy sampling) in the detection of resistance, specific genomic alterations, and minimum residual disease [102], as well as advancing its use in the prediction of treatment outcomes [103]. Controllability on the other hand is about the responsiveness of the system, i.e., the ability to steer it from one state to another. There are no general results for the controllability of nonlinear systems [95, 104]. However, conditions for small-time local controllability (STLC) have been worked out whereby the system can be dragged using admissible controls, in an arbitrarily small time, from any initial state to an end-state in a neighborhood that includes the initial state [105]. Here too, theoretical analysis of controllability based on mathematical models born out of simplifying assumptions on tumor growth dynamics may not yield significantly useful clinical insight on the controllability of the disease. Nevertheless, the theoretical results on STLC may be pragmatically applied by planning the desired state trajectory of the disease to be a sequence of desired disease states such that each next state can be reached in an arbitrarily small time using a small control (i.e., low drug doses), which causes a small perturbation of the tumor to keep its state within the neighborhood of the previous state. Although observability, controllability and reachability, which concerns the reachability of a system state starting from another state [96], may not be easily assessed for complex systems such as cancer, they, nevertheless, provide a theoretical reference for the choices of treatment response biomarkers, estimation models of disease states, and the treatment planning and adaptation schemes that are more likely to be effective in achieving a decisive management or cure of cancer.
| Why GenAI Is a Potential Catalyst for Effective Cancer Treatments? | ▴Top |
Current standard of cancer care involves the planning of fixed treatment schedules, where treatment cycles are carried out to their completion unless interrupted by events such as severe toxicity, infection or other complications. Treatment planning would in general be based on risk stratification and prognosis biomarkers, in addition to drawing on past experiences with similar cases and applicable institutional and community guidelines. Treatments are usually adjusted based on clinical guidelines and clinician’s experience in a reactive approach to treatment response. This entails ceding the oncologist’s initial advantage to cancer’s evolutionary dynamics to drive disease progression, which more often than not leads to therapeutic resistance, metastasis and death. These evolutionary dynamics are ultimately fed by the loss of cellular homeostasis, which relies on the cell signaling network to successfully orchestrate cellular activities and processes (e.g., growth, proliferation, survival, apoptosis and metabolism) and to regulate both inter-cellular and intra-cellular communications. Deregulated cellular functions ensue from genetic and epigenetic alterations and their evolving diversity, which underlie carcinogenesis and the successive acquisition of cancer hallmark capabilities [11-15]. These alterations affect canonical pathways such as cell growth, the cell cycle, and apoptosis [106], as well as reprogram metabolism [107], consequently enabling the survival and proliferation of cancer cells through the activation of oncogenic pathways and the abrogation of tumor suppression functions. Although targeted therapy has yielded significant clinical success in the treatment of cancer, it is often rendered ineffective by the evolving nature of cancer. For instance, a study on mutational burden in CRC reveals that at diagnosis, every DNA locus has a mutation in at least one cancer cell [108]. This deep reservoir of genetic aberrations combined with eco-evolutionary pressures on cancer cells under treatment feed tumor heterogeneity and phenotypic plasticity, leading, ultimately, to the emergence of therapeutic resistance. Furthermore, tumors are metabolically heterogenous and flexible, with evolving metabolic vulnerabilities that are challenging to target, providing metabolic avenues for therapeutic resistance [107]. This has led to the rise of combination therapy as an essential component of standard of care, whereby concurrent and/or sequential combinations of drugs are used to mitigate therapeutic resistance [109]. Although accelerated translational efforts are ongoing to leverage combination therapy, as illustrated by the thousands of ongoing clinical trials, this treatment paradigm needs further research on rationalizing drug combinations, as it is not feasible to study the near-infinite possible combinations of available drugs [110-115]. Furthermore, in order to achieve a desirable efficacy while limiting additive toxicity, scheduling combinations of drugs and their doses need to be optimized [115-119]. While combination therapy is a mainstay of current cancer treatments, the evolving nature of cancer has primed the rise of adaptive therapy as a treatment strategy conceived to thwart therapeutic resistance through treatment modulation based on monitored treatment response [89, 120-128], with the guidance of mathematical modeling [129-132]. Compared to fixed-schedule treatments, adaptive therapy provides potential advantages vis-a-vis treatment personalization, toxicity, and resistance management [39]. This is aligned with the gain in momentum for adaptive therapy, whereby increasing number of clinical trials are either planned or ongoing for various cancers, including melanoma, prostate and ovarian cancers [125, 126, 133, 134]. The joining of adaptive and combination therapies is a natural next step to leverage the expanding set of drug options becoming available to patients towards more effective treatments. In this respect, game theoretic mathematical models have been explored to design multi-drug adaptive therapy that mitigates therapeutic resistance through an appropriate timing of the switch between possible treatments (e.g., one drug, a drug combination or no treatment) based on the monitoring of tumor clonal composition [123]. Combination therapy involving adaptive switching between chemotherapy, immunotherapy and targeted therapy based on the repeated monitoring of tumor entropy has also been explored under the assumption that cell adaptive fitness imposes constraints on the possible trajectories of tumor growth [89, 135, 136]. One example clinical trial of adaptive combination therapy is the adaptive androgen deprivation therapy for metastatic castration sensitive prostate cancer (mCSPC) [126]. In this case, the adaptation heuristic for treatment stopping/resumption and the selective administration of new hormonal agent (NHA), luteinizing hormone releasing hormone (LHRH) or a combination thereof is based on repeatedly monitored prostate specific antigen (PSA) and testosterone levels, as well as imaging progression [126]. There are at least two fundamental challenges that need to be overcome in order to realize the full potential of adaptive therapy in delivering decisive cancer treatments. First, progress is needed regarding the discovery and validation of treatment response biomarkers that could provide an accurate observation of disease evolutionary dynamics. Second, robust and faithful reconstructions of the trajectories of cancer evolutionary dynamics using treatment response biomarkers is imperative to the synthesis of effective therapy adaptation strategies. GenAI holds a formidable potential to address these and other challenges by learning the relationships between the various dimensions of cancer complexity from clinical, pre-clinical and experimental studies on the treatment of cancer (Table 1) [39, 80, 121, 123, 126, 127, 128, 133, 137, 138]. This would facilitate the design of effective treatments by providing data-driven treatment response predictions and by assisting in the planning and adaptation of treatments [39]. In particular, GenAI can assist oncologists to overcome the information processing bottleneck associated with the limitations of human cognitive capacity [139], which arise when tackling high-dimensional, multivariate and complex treatment decision-making problems. These problems involve the consideration of a significantly large number of variables, including treatment response, toxicity, therapeutic options, past treatments, patient wishes, comorbidity and age, as well as financial and healthcare delivery environment’s constraints. The potential role of AI in this respect is also particularly pertinent to maintaining the efficacy of multidisciplinary tumor boards (MTBs) in providing timely, optimized and comprehensive treatment plans based on the analysis of big multimodal clinical, molecular and radiomic data, in the face of increasing patient caseload [137]. Indeed, while facing reliability challenges in their present forms, LLMs could be moderately helpful as decision-support tools for MTBs [138].
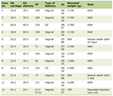 Click to view | Table 1. GenAI as a Catalyst for Effective Cancer Treatments |
For an explicit consideration of cancer evolutionary dynamics, disease state can be defined as the vector of tumor clonal frequencies, which may not be directly measurable and would have to be estimated from repeated sampling of treatment response biomarkers. These biomarkers may include imaging data, as well as relevant genetic, immunological and eco-evolutionary biomarkers that are clinically validated to reflect disease state for the cancer type at hand [140]. Although biomarker panels have been compiled for different cancer types [141, 142], further studies are needed to identify minimal sets of biomarkers, by cancer type, that are sufficient to recover disease states. For example, the combination of PSA and testosterone has been shown to be clinically feasible as a proxy for tumor burden to guide adaptive androgen depravation therapy of mCSPC [126]. However, these need to be complemented with additional biomarkers that carry information about the changing genetic alterations of cancer cells, the tumor microenvironment and immune response so as to enable a satisfactory observability of disease state variables, i.e., tumor clonal frequencies. Future advances in imaging [143], liquid biopsy (LB) [144, 145] and next-generation sequencing (NGS) techniques hold the promise for accurate and robust tracking of the evolving genotypic and phenotypic diversity of tumors.
The framing of cancer treatment as an adaptive control problem opens avenues for the use of multiple possible adaptive strategies [89]. One commonly used system control strategy is the classical proportional-integral-derivative (PID) controller [146-148]. Given the errors between desired and current disease states, PID feedback control is based on the contributions of the current error (proportional term), the average of past errors (integral term) and future errors (derivative term) to the control signal [146]. The relative contributions of these terms to control actions (i.e., doses of administered drugs) are specified using tunable gains. This simple and transparent construction, and the consequent ease of use do undoubtedly support the fact that PID control is the most widely used industrial control strategy [148]. The state-feedback nature of this strategy relies on the estimation of disease states from real-time repeated monitoring of treatment response. Extensive explorations have been undertaken on the applicability of LLMs’ learning and predictive capabilities to the estimation of disease states based on treatment response monitoring [37-39]. Both mathematical foundations of DL, which underlies LLMs, and technological advances in LB, imaging, and NGS for treatment response monitoring support the practical feasibility of LLM-based tracking of disease state dynamics [39]. However, its clinical feasibility will largely depend on the availability of large quality clinical datasets for training and regular fine-tuning of these dedicated LLMs [39]. The other aspect critical to the design of PID control strategies involves the tuning of PID gains to achieve a desired performance [146, 149]. Tuning approaches such as Ziegler-Nichols [146, 150, 151] methods, which involve probing the system using a step response or eliciting system oscillations, may not be feasible for cancer therapy due to the potential for severe toxicity and the long timescale of treatment response dynamics. While the adoption of PID control as the treatment adaptation strategy of choice is well supported by its proven performance, it is more intuitive, in the context of cancer therapy, that such adaptation be undertaken based on learned patterns from past effective treatments of phenotypically similar patients. The data-driven learning capabilities of GenAI could be leveraged for such purpose through the training of LLMs to generate self-tuned PID controls, which are driven by error signals between desired and observed disease states, within the context of past controls and control errors. The training of LLMs to generate controls that reflect PID control strategies require large quality training datasets, which is undeniably challenging. However, it is expected that in the long run the curation of quality treatment data for LLM training would be feasible, at least within the private confines of healthcare institutions, where the trained GenAI models are to be used to support the optimization of cancer treatments. One approach that would significantly reduce the need for large clinical datasets is to leverage transfer learning [152-154] by training LLMs on synthetically generated datasets associated with PID controls of clinically validated mathematical models of tumor evolutionary dynamics, followed by fine-tuning of the generative models using treatment data collected from patients that are phenotypically similar to the class of patients under treatment. However, while the often-tailored foci of mathematical tumor models on singular aspects (e.g., clonal dynamics, angiogenesis, and drug resistance) may represent a valuable source of synthetic training data, patient-derived xenografts (PDXs) are, aside from actual patients, the most pertinent sources of training data as will be discussed in the next section.
| Discussion | ▴Top |
The mathematically asserted property of deep neural network as universal approximators of nonlinear systems [155-158] is the cornerstone of LLM applicability to the modeling and prediction of cancer evolutionary dynamics. The practical feasibility of GenAI and their underlying LLMs as estimators of disease state dynamics and as learning models that can assist treatment decision-making is supported by deeply rooted results from research on the modeling of biological complexity [159], digital twins [160, 161], and rapid-learning healthcare [162-164]. In fact, LLMs are natural extensions and points of convergence of these approaches while being distinguished by their unique nature as standard general-purpose learning systems with a DL architecture that can be systematically trained using multimodal data to perform nearly all tasks relevant to cancer care, including diagnosis, prognostication and treatment decision-making [31, 32]. Despite the versatility of GenAI’s learning and reasoning capabilities in recasting an increasingly growing cancer data into actionable knowledge that could revolutionize cancer treatments, clinical translation and adoption of this technology face the challenges of training data availability, clinical evaluation, regulatory hurdles, and a host of ethical concerns, including bias, privacy, transparency, explainability and accountability [165-167]. Mitigating these concerns would need the development of bioethical frameworks for the responsible use of LLMs [167]. Principles of medical ethics such as beneficence, nonmaleficence, autonomy and justice have been proposed as possible pillars for frameworks of responsible LLM use in medicine [167]. These may guide the mitigation of the wide spectrum of ethical concerns from accountability to bias and transparency through specific actions such as using training data that represent the target patient population, tailoring predictions to specific groups of patients and flagging corresponding uncertainties [168], establishing AI governance committees to mitigate the dataset shift [80], and using human-in-the loop strategies as a guard rail against nonsensical output [169]. These mitigation approaches would inevitably need to be supported by advances in methods and techniques such as visualization, input feature importance, and counterfactual explanations, to improve the interpretability and explainability of GenAI black box models [170]. LLM hallucinations [171], i.e., generation of factually implausible outputs, and corresponding issues of reliability represent another concern, which has been the subject of various mitigation approaches, such as the use of retrieval-augmented generation (RAG) [172, 173]. Beyond the above discussed challenges, the integration of LLMs in real-world clinical settings will require judicious considerations of computational cost and the availability of technical expertise and resources [174].
GenAI training data may be sourced from patient treatments, clinical studies, PDXs, and clinically validated mathematical models. Although mathematical models of tumor growth represent a versatile approach to the generation of synthetic data, they are limited by their underlying assumptions and may not represent the biological complexity of tumors. Indeed, such models are often constructed for specific foci and under specific assumptions regarding various cancer aspects, such as genetic alterations, cancer metabolism, dysregulated signaling, cancer-immune cells’ interactions, and tumor physical properties, limiting as a result their capacity to yield a systematic span of cancer state dynamics. A more pertinent approach would be the use of PDX models to curate training datasets. Indeed, PDXs are often used for pre-clinical studies of drug combinations and resistance given their preservation of determinant features of patient tumors [175-183], making them ideal systems for the generation of big quality training data that span different types of cancer, as well as cover an arbitrarily large spectrum of genetic and phenotypic diversity within each cancer type. Given the critical role of data in GenAI-assisted cancer treatments, efforts to curate PDX and patient treatment data would be best undertaken through a dedicated international consortium, such as the international cancer genome consortium (ICGC), which would yield more diverse patient datasets and help mitigate the potential bias of GenAI-assisted treatment decision-making.
The transparently simple construction of PID controllers and their ubiquitous use in industry [146, 148] justify their consideration as a suitable control strategy for real-world clinical settings of cancer treatments. However, adaptive feedback control theory offers a wide spectrum of control strategies, which can in principle be applied to adaptive cancer therapy, including approaches from the three major controller classes of gain scheduling, self-tuning regulators and model-reference adaptive controllers [90-92, 184]. PID control embodies a reference design against which the performance and suitability of other complex strategies in these classes can be gauged, and its consideration would hence be most appropriate as a first choice for the nascent paradigm of adaptive cancer therapy. Beyond the design transparency of PID controllers, self-tuning PID controls [185-187], with tuning algorithms informed by patient-tailored predictive models of disease dynamics, would also offer a versatile yet still transparent design to deal with tumor heterogeneity manifested as varying nonlinearities of tumor growth dynamics across patients and time.
RCTs remain the gold standard to demonstrate safety, reliability and effectiveness of clinical interventions. Unfortunately, there is a dearth of prospective clinical studies of AI systems in healthcare [81], which is aligned with a noticeable “AI Chasm” separating the development of AI systems and their deployment in real-world clinical settings [188]. RCT reporting guidelines such as CONSORT-AI [189], DECIDE-AI [190] and SPIRIT-AI [191] have been introduced to help address this gap. These guidelines will inevitably inform the design of AI-assisted clinical studies by highlighting AI-related aspects that need attention. The persisting challenge of therapeutic resistance, the need to improve the QoL of cancer patients, and GenAI potential contribution to the mitigation of resource constraints, are likely to become prominent incentives for clinical institutions to engage in prospective studies of AI systems. However, this will require the engagement of pharmaceutical and AI companies, as well as the development of flexible, AI-pertinent regulatory approval guidelines of clinical interventions.
| Conclusions | ▴Top |
Despite the significant expansion of cancer therapeutic options, therapeutic resistance persists as a critical barrier to cancer long-term management and cure. Adaptive therapy, which may involve combination of multiple drugs, represent a plausible strategy to thwart or delay the onset of therapeutic resistance. Given the framing of cancer treatment as a control problem, data-driven GenAI learning and predictive capabilities have the potential to address the challenges facing the clinical realizability of adaptive therapy, including the accurate and reliable estimation of disease state and tumor growth dynamics. The realization of this potential is however contingent on the success of efforts aimed at curating large quality datasets for GenAI training and will require accelerated efforts of clinical evaluations supported by regulatory approval frameworks that must be flexible and considerate of AI unique challenges in real-world clinical settings.
Acknowledgments
None to declare
Financial Disclosure
None to declare
Conflict Interest
None to declare
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Early Breast Cancer Trialists' Collaborative g. Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22(8):1139-1150.
doi pubmed - Miyashita H, Kato S, Hong DS. KRAS G12C inhibitor combination therapies: current evidence and challenge. Front Oncol. 2024;14:1380584.
doi pubmed - Phillips C. FDA approves trastuzumab deruxtecan for any HER2-positive solid cancer. In: National Cancer Institut; 2024.
- Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, Gonzalez-Martin A, Jung KH, et al. Efficacy and safety of Trastuzumab Deruxtecan in patients with HER2-expressing solid tumors: Primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol. 2024;42(1):47-58.
doi pubmed - Planchard D, Brahmer JR, Yang JC, Chen YM, Lee KY, Suksombooncharoen T, Viglianti N, et al. Abstract CT572: Phase 1b dose-escalation and dose-expansion study evaluating trastuzumab deruxtecan (T-DXd) in combination with durvalumab and cisplatin, carboplatin, or pemetrexed in advanced or metastatic, HER2-overexpressing, nonsquamous non-small cell lung cancer (NSCLC): DESTINY-Lung03. Cancer Research. 2022;82(12_Supplement):CT572.
- Schwab ED, Pienta KJ. Cancer as a complex adaptive system. Med Hypotheses. 1996;47(3):235-241.
doi pubmed - Mallick P. Complexity and information: cancer as a multi-scale complex adaptive system. In: Physical sciences and engineering advances in life sciences and oncology: a WTEC global assessment. edn. Edited by: Janmey P, Fletcher D, Gerecht S, Levine R, Mallick P, McCarty O, Munn L, et al. Springer International Publishing; 2016: p. 5-29.
- Derbal Y. The adaptive complexity of cancer. Biomed Res Int. 2018;2018:5837235.
doi pubmed - Barker AD, Buetow K. Abstract SY36-03: Viewing cancer as a complex adaptive system and managing immunotherapy as “homeostatic reset”. Cancer Research. 2019;79(13_Supplement):SY36-03-SY36-03.
- Anderson A, Gillies R, Gatenby R. Cancer is complex and dynamicergo, dose optimization calls for mathematical modeling. The Cancer Letter. 2022.
- Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J Cell Sci. 2008;121(Suppl 1):1-84.
doi pubmed - Vogt PK. Cancer genes. West J Med. 1993;158(3):273-278.
pubmed - Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57-70.
doi pubmed - Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
doi pubmed - Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12(1):31-46.
doi pubmed - Derbal Y. Perspective on the dynamics of cancer. Theor Biol Med Model. 2017;14(1):18.
doi pubmed - Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539-545.
doi pubmed - Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991-998.
doi pubmed - Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565-1570.
doi pubmed - Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19-20):1267-1284.
doi pubmed - Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321-330.
doi pubmed - Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23-28.
doi pubmed - Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6(12):924-935.
doi pubmed - Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306-313.
doi pubmed - Greaves M. Nothing in cancer makes sense except. BMC Biol. 2018;16(1):22.
doi pubmed - Greaves M. Evolutionary determinants of cancer. Cancer Discov. 2015;5(8):806-820.
doi pubmed - Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, Kaiser L, et al. Attention is all you need. Advances in neural information processing systems. 2017;30:6000-6010.
- Gozalo-Brizuela R. A survey of Generative AI applications. Cornell University. 2023.
- Islam S, Rjoub G, Elmekki H, Bentahar J, Pedrycz W, Cohen R. Machine learning innovations in CPR: a comprehensive survey on enhanced resuscitation techniques. Artif Intell Rev. 2025;58(8):233.
doi pubmed - Khan S, Naseer M, Hayat M, Zamir SW, Khan FS, Shah M. Transformers in vision: a survey. ACM computing surveys (CSUR). 2022;54(10s):1-41.
- Saab K, Tu T, Weng WH, Tanno R, Stutz D, Wulczyn E, Zhang F, et al. Capabilities of gemini models in medicine. arXiv preprint. 2024.
- Yang L, Xu S, Sellergren A, Kohlberger T, Zhou Y, Ktena I, Kiraly A, et al. Advancing multimodal medical capabilities of Gemini. arXiv preprint. 2024.
- Katz U, Cohen E, Shachar E, Somer J, Fink A, Morse E, Shreiber B, et al. GPT versus resident physicians - a benchmark based on official board scores. NEJM AI. 2024;1(5):AIdbp2300192.
- Eriksen AV, Moller S, Ryg J. Use of GPT-4 to diagnose complex clinical cases. Massachusetts Medical Society. 2024;1:AIp2300031.
- Benary M, Wang XD, Schmidt M, Soll D, Hilfenhaus G, Nassir M, Sigler C, et al. Leveraging large language models for decision support in personalized oncology. JAMA Netw Open. 2023;6(11):e2343689.
doi pubmed - Carl N, Schramm F, Haggenmuller S, Kather JN, Hetz MJ, Wies C, Michel MS, et al. Large language model use in clinical oncology. NPJ Precis Oncol. 2024;8(1):240.
doi pubmed - Derbal Y. Adaptive cancer therapy in the age of generative artificial intelligence. Cancer Control. 2024;31:10732748241264704.
doi pubmed - Derbal Y. Adaptive treatment of metastatic prostate cancer using generative artificial intelligence. Clin Med Insights Oncol. 2025;19:11795549241311408.
doi pubmed - Derbal Y. Generative AI - assisted adaptive cancer therapy. Cancer Control. 2025;32:10732748251349919.
doi pubmed - Nagy M, Radakovich N, Nazha A. Machine learning in oncology: what should clinicians know? JCO Clin Cancer Inform. 2020;4:799-810.
doi pubmed - Hong JC, Eclov NCW, Dalal NH, Thomas SM, Stephens SJ, Malicki M, Shields S, et al. System for high-intensity evaluation during radiation therapy (SHIELD-RT): a prospective randomized study of machine learning-directed clinical evaluations during radiation and chemoradiation. J Clin Oncol. 2020;38(31):3652-3661.
doi pubmed - McIntosh C, Conroy L, Tjong MC, Craig T, Bayley A, Catton C, Gospodarowicz M, et al. Clinical integration of machine learning for curative-intent radiation treatment of patients with prostate cancer. Nat Med. 2021;27(6):999-1005.
doi pubmed - Sammut SJ, Crispin-Ortuzar M, Chin SF, Provenzano E, Bardwell HA, Ma W, Cope W, et al. Multi-omic machine learning predictor of breast cancer therapy response. Nature. 2022;601(7894):623-629.
doi pubmed - Derbal Y. Can artificial intelligence improve cancer treatments? Health Informatics J. 2022;28(2):14604582221102314.
doi pubmed - Bera K, Braman N, Gupta A, Velcheti V, Madabhushi A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol. 2022;19(2):132-146.
doi pubmed - Boehm KM, Aherne EA, Ellenson L, Nikolovski I, Alghamdi M, Vazquez-Garcia I, Zamarin D, et al. Multimodal data integration using machine learning improves risk stratification of high-grade serous ovarian cancer. Nat Cancer. 2022;3(6):723-733.
doi pubmed - Swanson K, Wu E, Zhang A, Alizadeh AA, Zou J. From patterns to patients: Advances in clinical machine learning for cancer diagnosis, prognosis, and treatment. Cell. 2023;186(8):1772-1791.
doi pubmed - Boehm KM, Khosravi P, Vanguri R, Gao J, Shah SP. Harnessing multimodal data integration to advance precision oncology. Nat Rev Cancer. 2022;22(2):114-126.
doi pubmed - Jiao W, Atwal G, Polak P, Karlic R, Cuppen E, Subtypes PT, Clinical Translation Working G, et al. A deep learning system accurately classifies primary and metastatic cancers using passenger mutation patterns. Nat Commun. 2020;11(1):728.
doi pubmed - Kann BH, Hosny A, Aerts H. Artificial intelligence for clinical oncology. Cancer Cell. 2021;39(7):916-927.
doi pubmed - Azuaje F. Artificial intelligence for precision oncology: beyond patient stratification. NPJ Precis Oncol. 2019;3:6.
doi pubmed - Shreve JT, Khanani SA, Haddad TC. Artificial intelligence in oncology: current capabilities, future opportunities, and ethical considerations. Am Soc Clin Oncol Educ Book. 2022;42:1-10.
doi pubmed - Senthil Kumar K, Miskovic V, Blasiak A, Sundar R, Pedrocchi ALG, Pearson AT, Prelaj A, et al. Artificial intelligence in clinical oncology: from data to digital pathology and treatment. Am Soc Clin Oncol Educ Book. 2023;43:e390084.
doi pubmed - Luchini C, Pea A, Scarpa A. Artificial intelligence in oncology: current applications and future perspectives. Br J Cancer. 2022;126(1):4-9.
doi pubmed - Ho D. Artificial intelligence in cancer therapy. Science. 2020;367(6481):982-983.
doi pubmed - Lotter W, Hassett MJ, Schultz N, Kehl KL, Van Allen EM, Cerami E. Artificial intelligence in oncology: current landscape, challenges, and future directions. Cancer Discov. 2024;14(5):711-726.
doi pubmed - Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. [https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices].
- Sasaki M, Tozaki M, Rodriguez-Ruiz A, Yotsumoto D, Ichiki Y, Terawaki A, Oosako S, et al. Artificial intelligence for breast cancer detection in mammography: experience of use of the ScreenPoint Medical Transpara system in 310 Japanese women. Breast Cancer. 2020;27(4):642-651.
doi pubmed - Seager A, Sharp L, Hampton JS, Neilson LJ, Lee TJW, Brand A, Evans R, et al. Trial protocol for COLO-DETECT: A randomized controlled trial of lesion detection comparing colonoscopy assisted by the GI Genius artificial intelligence endoscopy module with standard colonoscopy. Colorectal Dis. 2022;24(10):1227-1237.
doi pubmed - Cherubini A, Dinh NN. A review of the technology, training, and assessment methods for the first real-time AI-enhanced medical device for endoscopy. Bioengineering (Basel). 2023;10(4):404.
doi pubmed - Raciti P, Sue J, Ceballos R, Godrich R, Kunz JD, Kapur S, Reuter V, et al. Novel artificial intelligence system increases the detection of prostate cancer in whole slide images of core needle biopsies. Mod Pathol. 2020;33(10):2058-2066.
doi pubmed - Placido D, Yuan B, Hjaltelin JX, Zheng C, Haue AD, Chmura PJ, Yuan C, et al. A deep learning algorithm to predict risk of pancreatic cancer from disease trajectories. Nat Med. 2023;29(5):1113-1122.
doi pubmed - Rafique R, Islam SMR, Kazi JU. Machine learning in the prediction of cancer therapy. Comput Struct Biotechnol J. 2021;19:4003-4017.
doi pubmed - Kendrick J, Francis R, Hassan GM, Rowshanfarzad P, Jeraj R, Kasisi C, Rusanov B, et al. Radiomics for identification and prediction in metastatic prostate cancer: a review of studies. Front Oncol. 2021;11:771787.
doi pubmed - Lu L, Dercle L, Zhao B, Schwartz LH. Deep learning for the prediction of early on-treatment response in metastatic colorectal cancer from serial medical imaging. Nat Commun. 2021;12(1):6654.
doi pubmed - Nagpal K, Foote D, Tan F, Liu Y, Chen PC, Steiner DF, Manoj N, et al. Development and Validation of a Deep Learning Algorithm for Gleason Grading of Prostate Cancer From Biopsy Specimens. JAMA Oncol. 2020;6(9):1372-1380.
doi pubmed - Courtiol P, Maussion C, Moarii M, Pronier E, Pilcer S, Sefta M, Manceron P, et al. Deep learning-based classification of mesothelioma improves prediction of patient outcome. Nat Med. 2019;25(10):1519-1525.
doi pubmed - Bychkov D, Linder N, Turkki R, Nordling S, Kovanen PE, Verrill C, Walliander M, et al. Deep learning based tissue analysis predicts outcome in colorectal cancer. Sci Rep. 2018;8(1):3395.
doi pubmed - Xu Y, Hosny A, Zeleznik R, Parmar C, Coroller T, Franco I, Mak RH, et al. Deep learning predicts lung cancer treatment response from serial medical imaging. Clin Cancer Res. 2019;25(11):3266-3275.
doi pubmed - Shi J, Alagoz O, Erenay FS, Su Q. A survey of optimization models on cancer chemotherapy treatment planning. Annals of Operations Research. 2014;221:331-356.
- Blasiak A, Khong J, Kee T. CURATE.AI: optimizing personalized medicine with artificial intelligence. SLAS Technol. 2020;25(2):95-105.
doi pubmed - Liu S, Pastor-Serrano O, Chen Y, Gopaulchan M, Liang W, Buyyounouski M, Pollom E, et al. Automated radiotherapy treatment planning guided by GPT-4Vision. ArXiv. 2025.
pubmed - Haemmerli J, Sveikata L, Nouri A, May A, Egervari K, Freyschlag C, Lobrinus JA, et al. ChatGPT in glioma adjuvant therapy decision making: ready to assume the role of a doctor in the tumour board? BMJ Health Care Inform. 2023;30(1).
doi pubmed - Rajendran P, Chen Y, Qiu L, Niedermayr T, Liu W, Buyyounouski M, Bagshaw H, et al. Autodelineation of treatment target volume for radiation therapy using large language model-aided multimodal learning. Int J Radiat Oncol Biol Phys. 2025;121(1):230-240.
doi pubmed - Rajendran P, Yang Y, Niedermayr TR, Gensheimer M, Beadle B, Le QT, Xing L, et al. Large language model-augmented auto-delineation of treatment target volume in radiation therapy. ArXiv. 2024.
pubmed - Glatzer M, Panje CM, Siren C, Cihoric N, Putora PM. Decision making criteria in oncology. Oncology. 2020;98(6):370-378.
doi pubmed - Iseli T, Fischer GF, Panje CM, Glatzer M, Hundsberger T, Rothermundt C, Schmidt B, et al. Insular decision criteria in clinical practice: analysis of decision-making in oncology. Oncology. 2020;98(6):438-444.
doi pubmed - Harish V, Morgado F, Stern AD, Das S. Artificial intelligence and clinical decision making: the new nature of medical uncertainty. Acad Med. 2021;96(1):31-36.
doi pubmed - Angus DC. Randomized clinical trials of artificial intelligence. JAMA. 2020;323(11):1043-1045.
doi pubmed - Finlayson SG, Subbaswamy A, Singh K, Bowers J, Kupke A, Zittrain J, Kohane IS, et al. The clinician and dataset shift in artificial intelligence. N Engl J Med. 2021;385(3):283-286.
doi pubmed - Kelly CJ, Karthikesalingam A, Suleyman M, Corrado G, King D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019;17(1):195.
doi pubmed - Marra A, Morganti S, Pareja F, Campanella G, Bibeau F, Fuchs T, Loda M, et al. Artificial intelligence entering the pathology arena in oncology: current applications and future perspectives. Ann Oncol. 2025;36(7):712-725.
doi pubmed - Iannantuono GM, Bracken-Clarke D, Floudas CS, Roselli M, Gulley JL, Karzai F. Applications of large language models in cancer care: current evidence and future perspectives. Front Oncol. 2023;13:1268915.
doi pubmed - Clusmann J, Kolbinger FR, Muti HS, Carrero ZI, Eckardt JN, Laleh NG, Loffler CML, et al. The future landscape of large language models in medicine. Commun Med (Lond). 2023;3(1):141.
doi pubmed - Stalp JL, Denecke A, Jentschke M, Hillemanns P, Klapdor R. Quality of ChatGPT-generated therapy recommendations for breast cancer treatment in gynecology. Curr Oncol. 2024;31(7):3845-3854.
doi pubmed - Touvron H, Martin L, Stone K, Albert P, Almahairi A, Babaei Y, Bashlykov N, et al. Llama 2: Open foundation and fine-tuned chat models. arXiv preprint. 2023.
pubmed - Hager P, Jungmann F, Holland R, Bhagat K, Hubrecht I, Knauer M, Vielhauer J, et al. Evaluation and mitigation of the limitations of large language models in clinical decision-making. Nat Med. 2024;30(9):2613-2622.
doi pubmed - Schulte B. Capacity of ChatGPT to identify guideline-based treatments for advanced solid tumors. Cureus. 2023;15(4):e37938.
doi pubmed - Derbal Y. Adaptive control of tumor growth. Cancer Control. 2024;31:10732748241230869.
doi pubmed - Sastry S, Bodson M, Bartram JF. Adaptive control: stability, convergence, and robustness. In: Acoustical Society of America; 1990.
- Astrom KJ, Wittenmark B. Adaptive control: courier corporation. 2013.
- Ioannou PA, Sun J. Robust adaptive control: courier corporation. 2012.
- Beckman RA, Kareva I, Adler FR. How should cancer models be constructed? Cancer Control. 2020;27(1):1073274820962008.
doi pubmed - Griffith EW, Kumar KS. On the observability of nonlinear systems: I. Journal of Mathematical Analysis and Applications. 1971;35(1):135-147.
- Hermann R, Krener A. Nonlinear controllability and observability. IEEE Transactions on Automatic Control. 1977;22(5):728-740.
- Sontag ED. Controllability is harder to decide than accessibility. SIAM journal on control and optimization. 1988;26(5):1106-1118.
- Sussmann HJ. A general theorem on local controllability. SIAM Journal on Control and Optimization. 1987;25(1):158-194
- Liu YY, Slotine JJ, Barabasi AL. Observability of complex systems. Proc Natl Acad Sci U S A. 2013;110(7):2460-2465.
doi pubmed - Yamamoto Y, Sugiura I. Some sufficient conditions for the observability of nonlinear systems. Journal of Optimization Theory and Applications. 1974;13:660-669.
- Lecca P, Re A. Identifying necessary and sufficient conditions for the observability of models of biochemical processes. Biophys Chem. 2019;254:106257.
doi pubmed - Villaverde AF. Observability and structural identifiability of nonlinear biological systems. Complexity. 2019;2019(1):8497093.
- Bartolomucci A, Nobrega M, Ferrier T, Dickinson K, Kaorey N, Nadeau A, Castillo A, et al. Circulating tumor DNA to monitor treatment response in solid tumors and advance precision oncology. NPJ Precis Oncol. 2025;9(1):84.
doi pubmed - Gouda MA, Huang HJ, Piha-Paul SA, Call SG, Karp DD, Fu S, Naing A, et al. Longitudinal monitoring of circulating tumor DNA to predict treatment outcomes in advanced cancers. JCO Precis Oncol. 2022;6:e2100512.
doi pubmed - Diaz-Seoane S, Barreiro Blas A, Villaverde AF. Controllability and accessibility analysis of nonlinear biosystems. Comput Methods Programs Biomed. 2023;242:107837.
doi pubmed - Lewis AD. A brief on controllability of nonlinear systems. Available from: https://mast.queensu.ca/∼andrew/notes/pdf/2001a.pdf.
- Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, Dimitriadoy S, et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell. 2018;173(2):321-337.e310.
doi pubmed - Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368(6487):eaaw5473.
doi pubmed - Loeb LA, Kohrn BF, Loubet-Senear KJ, Dunn YJ, Ahn EH, O'Sullivan JN, Salk JJ, et al. Extensive subclonal mutational diversity in human colorectal cancer and its significance. Proc Natl Acad Sci U S A. 2019;116(52):26863-26872.
doi pubmed - Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043.
doi pubmed - Rationalizing combination therapies. Nat Med. 2017;23(10):1113.
doi pubmed - Bozic I, Reiter JG, Allen B, Antal T, Chatterjee K, Shah P, Moon YS, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747.
doi pubmed - Day D, Siu LL. Approaches to modernize the combination drug development paradigm. Genome Med. 2016;8(1):115.
doi pubmed - Boshuizen J, Peeper DS. Rational Cancer Treatment Combinations: An Urgent Clinical Need. Mol Cell. 2020;78(6):1002-1018.
doi pubmed - Jin H, Wang L, Bernards R. Rational combinations of targeted cancer therapies: background, advances and challenges. Nat Rev Drug Discov. 2023;22(3):213-234.
doi pubmed - Lopez JS, Banerji U. Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol. 2017;14(1):57-66.
doi pubmed - Wu L, Leng D, Cun D, Foged C, Yang M. Advances in combination therapy of lung cancer: Rationales, delivery technologies and dosage regimens. J Control Release. 2017;260:78-91.
doi pubmed - Lee MJ, Ye AS, Gardino AK, Heijink AM, Sorger PK, MacBeath G, Yaffe MB. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149(4):780-794.
doi pubmed - Park SR, Davis M, Doroshow JH, Kummar S. Safety and feasibility of targeted agent combinations in solid tumours. Nat Rev Clin Oncol. 2013;10(3):154-168.
doi pubmed - Soria JC, Massard C, Izzedine H. From theoretical synergy to clinical supra-additive toxicity. J Clin Oncol. 2009;27(9):1359-1361.
doi pubmed - Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res. 2009;69(11):4894-4903.
doi pubmed - Enriquez-Navas PM, Wojtkowiak JW, Gatenby RA. Application of evolutionary principles to cancer therapy. Cancer Res. 2015;75(22):4675-4680.
doi pubmed - Gatenby RA, Brown JS. Integrating evolutionary dynamics into cancer therapy. Nat Rev Clin Oncol. 2020;17(11):675-686.
doi pubmed - West J, You L, Zhang J, Gatenby RA, Brown JS, Newton PK, Anderson ARA. Towards multidrug adaptive therapy. Cancer Res. 2020;80(7):1578-1589.
doi pubmed - Ma Y, Newton PK. Role of synergy and antagonism in designing multidrug adaptive chemotherapy schedules. Phys Rev E. 2021;103(3-1):032408.
doi pubmed - Zhang J, Cunningham J, Brown J, Gatenby R. Evolution-based mathematical models significantly prolong response to abiraterone in metastatic castrate-resistant prostate cancer and identify strategies to further improve outcomes. Elife. 2022;11.
doi pubmed - Zhang J, Gallaher J, Cunningham JJ, Choi JW, Ionescu F, Chatwal MS, Jain R, et al. A phase 1b adaptive androgen deprivation therapy trial in metastatic castration sensitive prostate cancer. Cancers (Basel). 2022;14(21).
doi pubmed - Hockings H, Lakatos E, Huang W, Mossner M, Khan MA, Bakali N, McDermott J, et al. Adaptive Therapy Exploits Fitness Deficits in Chemotherapy-Resistant Ovarian Cancer to Achieve Long-Term Tumor Control. Cancer Res. 2025.
doi pubmed - Zhang J, Cunningham JJ, Brown JS, Gatenby RA. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat Commun. 2017;8(1):1816.
doi pubmed - Stankova K, Brown JS, Dalton WS, Gatenby RA. Optimizing Cancer Treatment Using Game Theory: A Review. JAMA Oncol. 2019;5(1):96-103.
doi pubmed - Cunningham J, Thuijsman F, Peeters R, Viossat Y, Brown J, Gatenby R, Stankova K. Optimal control to reach eco-evolutionary stability in metastatic castrate-resistant prostate cancer. PLoS One. 2020;15(12):e0243386.
doi pubmed - Gluzman M, Scott JG, Vladimirsky A. Optimizing adaptive cancer therapy: dynamic programming and evolutionary game theory. Proc Biol Sci. 2020;287(1925):20192454.
doi pubmed - West J, Adler F, Gallaher J, Strobl M, Brady-Nicholls R, Brown J, Roberson-Tessi M, et al. A survey of open questions in adaptive therapy: Bridging mathematics and clinical translation. Elife. 2023;12.
doi pubmed - Circulating tumour DNA guided adaptive BRAF and MEK inhibitor therapy. [https://clinicaltrials.gov/study/NCT06470880].
- A multicentre phase II randomised controlled trial to evaluate the efficacy of adaptive therapy (AT) with carboplatin, based on changes in CA125, in patients with relapsed platinum-sensitive high grade serous or high grade endometrioid ovarian cancer. [https://clinicaltrials.gov/study/NCT05080556].
- Derbal Y. Cell adaptive fitness and cancer evolutionary dynamics. Cancer Inform. 2023;22:11769351231154679.
doi pubmed - Derbal Y. On signaling dysregulation in cancer. In: 2021 International Conference on Computational Science and Computational Intelligence: 2021. Las Vegas, USA. 2021; p. 318-323.
- Nardone V, Marmorino F, Germani MM, Cichowska-Cwalinska N, Menditti VS, Gallo P, Studiale V, et al. The role of artificial intelligence on tumor boards: perspectives from surgeons, medical oncologists and radiation oncologists. Curr Oncol. 2024;31(9):4984-5007.
doi pubmed - Ammo T, Guillaume VGJ, Hofmann UK, Ulmer NM, Buenting N, Laenger F, Beier JP, et al. Evaluating ChatGPT-4o as a decision support tool in multidisciplinary sarcoma tumor boards: heterogeneous performance across various specialties. Front Oncol. 2024;14:1526288.
doi pubmed - Halford GS, Baker R, McCredden JE, Bain JD. How many variables can humans process? Psychol Sci. 2005;16(1):70-76.
doi pubmed - Passaro A, Al Bakir M, Hamilton EG, Diehn M, Andre F, Roy-Chowdhuri S, Mountzios G, et al. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell. 2024;187(7):1617-1635.
doi pubmed - Zhou Y, Tao L, Qiu J, Xu J, Yang X, Zhang Y, Tian X, et al. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct Target Ther. 2024;9(1):132.
doi pubmed - Ma L, Guo H, Zhao Y, Liu Z, Wang C, Bu J, Sun T, et al. Liquid biopsy in cancer current: status, challenges and future prospects. Signal Transduct Target Ther. 2024;9(1):336.
doi pubmed - O'Connor JP, Aboagye EO, Adams JE, Aerts HJ, Barrington SF, Beer AJ, Boellaard R, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14(3):169-186.
doi pubmed - Alix-Panabieres C, Pantel K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021;11(4):858-873.
doi pubmed - Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18(5):297-312.
doi pubmed - Astr0m KJ, Murray R. Feedback systems: an introduction for scientists and engineers: Princeton university press; 2021.
- Bennett S. The past of PID controllers. Annual reviews in control. 2001;25:43-53.
- Li Y, Ang KH, Chong GC. Patents, software, and hardware for PID control: an overview and analysis of the current art. IEEE Control Systems Magazine. 2006;26(1):42-54.
- Astrom KJ, Hagglund T. Advanced PID control: ISA-The Instrumentation, Systems and Automation Society. 2006.
- Ziegler JG, Nichols NB. Optimum settings for automatic controllers. Transactions of the American society of mechanical engineers. 1942;64(8):759-765.
- Hang CC, Astrom KJ, Ho WK. Refinements of the Ziegler-Nichols tuning formula. In: IEE Proceedings D (Control Theory and Applications): 1991. IET. 1991; p. 111-118.
- Weiss K, Khoshgoftaar TM, Wang D. A survey of transfer learning. Journal of Big data. 2016;3:1-40.
- Pan SJ, Yang Q. A survey on transfer learning. IEEE Transactions on knowledge and data engineering. 2009;22(10):1345-1359.
- Torrey L, Shavlik J. Transfer learning. In: Handbook of research on machine learning applications and trends: algorithms, methods, and techniques. IGI global; 2010: p. 242-264.
- Hecht N. Theory of the backpropagation neural network. In: International 1989 Joint Conference on Neural Networks. 1989;591:593-605.
- Hornik K, Stinchcombe M, White H. Multilayer feedforward networks are universal approximators. Neural Networks. 1989;2(5):359-366.
- Kreinovich V. Arbitrary nonlinearity is sufficient to represent all functions by neural networks: A theorem. Neural Networks. 1991;4:381-383.
- Cotter NE. The Stone-Weierstrass theorem and its application to neural networks. IEEE Trans Neural Netw. 1990;1(4):290-295.
doi pubmed - Derbal Y. On modeling of living organisms using hierarchical coarse-graining abstractions of knowledge. Journal of Biological Systems. 2013;21:1350008.
- Glaessgen E, Stargel D. The digital twin paradigm for future NASA and US Air Force vehicles. In: 53rd AIAA/ASME/ASCE/AHS/ASC structures, structural dynamics and materials conference 20th AIAA/ASME/AHS adaptive structures conference, 14th AIAA. 2012;2012:1818.
- Hernandez-Boussard T, Macklin P, Greenspan EJ, Gryshuk AL, Stahlberg E, Syeda-Mahmood T, Shmulevich I. Digital twins for predictive oncology will be a paradigm shift for precision cancer care. Nat Med. 2021;27(12):2065-2066.
doi pubmed - Yu PP. Knowledge bases, clinical decision support systems, and rapid learning in oncology. J Oncol Pract. 2015;11(2):e206-211.
doi pubmed - Abernethy AP, Etheredge LM, Ganz PA, Wallace P, German RR, Neti C, Bach PB, et al. Rapid-learning system for cancer care. J Clin Oncol. 2010;28(27):4268-4274.
doi pubmed - Shrager J, Tenenbaum JM. Rapid learning for precision oncology. Nat Rev Clin Oncol. 2014;11(2):109-118.
doi pubmed - Haltaufderheide J, Ranisch R. The ethics of ChatGPT in medicine and healthcare: a systematic review on Large Language Models (LLMs). NPJ Digit Med. 2024;7(1):183.
doi pubmed - Li H, Moon JT, Purkayastha S, Celi LA, Trivedi H, Gichoya JW. Ethics of large language models in medicine and medical research. Lancet Digit Health. 2023;5(6):e333-e335.
doi pubmed - Ong JCL, Chang SYH, William W, Butte AJ, Shah NH, Chew LST, Liu N, et al. Medical ethics of large language models in medicine. NEJM AI. 2024;1(7):AIra2400038.
- Parikh RB, Teeple S, Navathe AS. Addressing bias in artificial intelligence in health care. JAMA. 2019;322(24):2377-2378.
doi pubmed - Shu L, He Q, Yan B, Wu D, Wang M, Wang C, Zhang L. Human-in-the-loop: Human involvement in enhancing medical inquiry performance in large language models. Allergy. 2024;79(5):1348-1351.
doi pubmed - Hassija V, Chamola V, Mahapatra A, Singal A, Goel D, Huang K, Scardapane S, et al. Interpreting black-box models: a review on explainable artificial intelligence. Cognitive Computation. 2024;16(1):45-74.
- Huang L, Yu W, Ma W, Zhong W, Feng Z, Wang H, Chen Q, et al. A survey on hallucination in large language models: Principles, taxonomy, challenges, and open questions. ACM Transactions on Information Systems. 2023.
- Tonmoy S, Zaman S, Jain V, Rani A, Rawte V, Chadha A, Das A. A comprehensive survey of hallucination mitigation techniques in large language models. arXiv preprint. 2024.
- Kang H, Ni J, Yao H. Ever: Mitigating hallucination in large language models through real-time verification and rectification. arXiv preprint. 2023.
- Dennstadt F, Hastings J, Putora PM, Schmerder M, Cihoric N. Implementing large language models in healthcare while balancing control, collaboration, costs and security. NPJ Digit Med. 2025;8(1):143.
doi pubmed - Marangoni E, Vincent-Salomon A, Auger N, Degeorges A, Assayag F, de Cremoux P, de Plater L, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13(13):3989-3998.
doi pubmed - Weroha SJ, Becker MA, Enderica-Gonzalez S, Harrington SC, Oberg AL, Maurer MJ, Perkins SE, et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res. 2014;20(5):1288-1297.
doi pubmed - Zanella ER, Grassi E, Trusolino L. Towards precision oncology with patient-derived xenografts. Nat Rev Clin Oncol. 2022;19(11):719-732.
doi pubmed - Izumchenko E, Paz K, Ciznadija D, Sloma I, Katz A, Vasquez-Dunddel D, Ben-Zvi I, et al. Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann Oncol. 2017;28(10):2595-2605.
doi pubmed - Yan C, Brunson DC, Tang Q, Do D, Iftimia NA, Moore JC, Hayes MN, et al. Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell. 2019;177(7):1903-1914.e1914.
doi pubmed - Liu Y, Wu W, Cai C, Zhang H, Shen H, Han Y. Patient-derived xenograft models in cancer therapy: technologies and applications. Signal Transduct Target Ther. 2023;8(1):160.
doi pubmed - Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, Clarke RB, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998-1013.
doi pubmed - Bruna A, Rueda OM, Greenwood W, Batra AS, Callari M, Batra RN, Pogrebniak K, et al. A biobank of breast cancer explants with preserved intra-tumor heterogeneity to screen anticancer compounds. Cell. 2016;167(1):260-274.e222.
doi pubmed - Stebbing J, Paz K, Schwartz GK, Wexler LH, Maki R, Pollock RE, Morris R, et al. Patient-derived xenografts for individualized care in advanced sarcoma. Cancer. 2014;120(13):2006-2015.
doi pubmed - Goodwin GC, Sin KS. Adaptive filtering prediction and control: Courier Corporation. 2014.
- Hernandez-Alvarado R, Garcia-Valdovinos LG, Salgado-Jimenez T, Gomez-Espinosa A, Fonseca-Navarro F. Neural network-based self-tuning PID control for underwater vehicles. Sensors (Basel). 2016;16(9):1429.
doi pubmed - Rodriguez-Abreo O, Rodriguez-Resendiz J, Fuentes-Silva C, Hernandez-Alvarado R, Falcon MDCPT. Self-tuning neural network PID with dynamic response control. IEEE Access 2021;9:65206-65215.
- Clarke D. The application of self-tuning control. Transactions of the Institute of Measurement and Control. 1983;5(2):59-69.
- Keane PA, Topol EJ. With an eye to AI and autonomous diagnosis. NPJ Digit Med. 2018;1:40.
doi pubmed - Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK, Spirit AI, ONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Lancet Digit Health. 2020;2(10):e537-e548.
doi pubmed - Vasey B, Nagendran M, Campbell B, Clifton DA, Collins GS, Denaxas S, Denniston AK, et al. Reporting guideline for the early stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. BMJ. 2022;377:e070904.
doi pubmed - Cruz Rivera S, Liu X, Chan AW, Denniston AK, Calvert MJ, Spirit AI, CONSORT-AI Working Group. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Lancet Digit Health. 2020;2(10):e549-e560.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
AI in Clinical Medicine is published by Elmer Press Inc.