| AI in Clinical Medicine, ISSN 0000-0000 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, AI Clin Med and Elmer Press Inc |
| Journal website https://aicm.elmerpub.com |
Review
Volume 1, 2025, e6
Top Ten Transformative Impacts of Artificial Intelligence on Life Sciences
Geng Zanga, c, Zilong Wangb, c
aResearch Centre, Centre Hospitalier de l’Universite de Montreal, Montreal, QC H2X 0A9, Canada
bSchool of Computer Science, McGill University, Montreal, QC H3A 2A7, Canada
cCorresponding Authors: Geng Zang, Research Centre, Centre Hospitalier de l’Universite de Montreal, Montreal, QC H2X 0A9, Canada; Zilong Wang, School of Computer Science, McGill University, Montreal, QC H3A 2A7, Canada
Manuscript submitted July 20, 2025, accepted July 28, 2025, published online August 7, 2025
Short title: Transformative Impacts of AI on Life Sciences
doi: https://doi.org/10.14740/aicm6
- Abstract
- Introduction
- Drug Discovery and Development
- Precision Medicine
- Medical Imaging and Diagnostics
- Genomics and Omics Analysis
- Clinical Decision Support
- Biological Data Integration and KGs
- Synthetic Biology and Protein Engineering
- Clinical Trial Optimization
- Public Health Surveillance and Epidemiology
- Laboratory Automation and Robotics
- Conclusion
- References
| Abstract | ▴Top |
Artificial intelligence (AI) is rapidly transforming the life sciences, revolutionizing biomedical research, diagnostics, therapeutics, and public health. Its ability to analyze complex data and uncover hidden patterns enables new solutions to long-standing biological challenges. This comprehensive review aims to identify and summarize the top 10 most impactful applications of AI across the life sciences, showcasing how AI technologies are reshaping key areas from drug discovery to public health surveillance. A narrative review approach was employed to synthesize recent advances and landmark developments across 10 major domains where AI has demonstrated transformative impact. Literature and case studies were examined to highlight the integration of AI tools in both research and clinical practice. Key areas of AI impact include: 1) drug discovery and development via predictive modeling and molecular generation, exemplified by AlphaFold; 2) precision medicine through integration of multi-omics and clinical data; 3) AI-assisted diagnostics in radiology and pathology; 4) omics data interpretation to uncover biomarkers and disease mechanisms; 5) clinical decision support using real-time data synthesis; 6) knowledge graphs for systems biology and drug-disease-gene relationships; 7) protein and enzyme design in synthetic biology; 8) clinical trial optimization via improved recruitment and risk prediction; 9) AI-driven public health surveillance; and 10) laboratory automation to enhance reproducibility and throughput. AI is not only accelerating discovery and development across the life sciences but also is fundamentally transforming how biomedical science is conducted. As AI technologies continue to evolve, they are poised to become indispensable tools for advancing healthcare innovation and addressing future biological challenges.
Keywords: Artificial intelligence; Life sciences; Drug discovery; Precision medicine; Medical imaging; Omics integration; Clinical decision support; Laboratory automation
| Introduction | ▴Top |
Artificial intelligence (AI) is revolutionizing life sciences, driving unprecedented advancements in drug discovery, diagnostics, personalized medicine, and biological research. By leveraging machine learning (ML), particularly deep learning and natural language processing (NLP), AI enables researchers to analyze vast datasets, uncover patterns, and accelerate innovation with precision and efficiency. From predicting protein structures to optimizing clinical trials, AI’s transformative potential is reshaping how we understand and address complex biological challenges. This introduction explores the profound impacts of AI on life sciences, highlighting its role in enhancing research, improving patient outcomes, and reducing costs.
In drug discovery, AI models like AlphaFold have solved decades-old problems, such as protein folding, enabling faster therapeutic development [1]. In diagnostics, AI-powered tools achieve near-human accuracy in detecting diseases from medical imaging, as demonstrated by studies on AI-driven radiology [2]. Personalized medicine benefits from AI’s ability to analyze genomic data, tailoring treatments to individual patients [3]. Additionally, AI streamlines clinical trials by identifying suitable candidates and predicting outcomes, reducing time and costs [4].
Recent advancements underscore AI’s growing influence. For instance, generative AI models are designing novel molecules for drug development [5]. Meanwhile, AI-driven bioinformatics tools are decoding complex genomic datasets, advancing precision medicine [6]. These innovations highlight AI’s potential to transform life sciences, paving the way for breakthroughs that were once unimaginable.
This article explores the top 10 impacts of AI, showcasing its role in shaping the future of healthcare and biological discovery. AI tools like ChatGPT, DeepSeek, and Grok are transforming life sciences across 10 key domains (Fig. 1 and Table 1). We highlight the most common areas of impact identified by these models, ranked by frequency and relevance. AI accelerates drug discovery, predicts protein structures, and personalizes care through precision medicine. In medical imaging, AI matches or surpasses human diagnostics, while in genomics and omics, it deciphers complex biological data. Clinical decision support is enhanced by AI’s integration of electronic health record (EHR) and predictive analytics. Knowledge graphs (KGs) uncover hidden gene-disease-drug links, and AI advances synthetic biology via protein and enzyme design. AI optimizes clinical trials by improving recruitment and outcome prediction. Lastly, lab automation powered by AI boosts research speed, scalability, and reproducibility (Fig. 2).
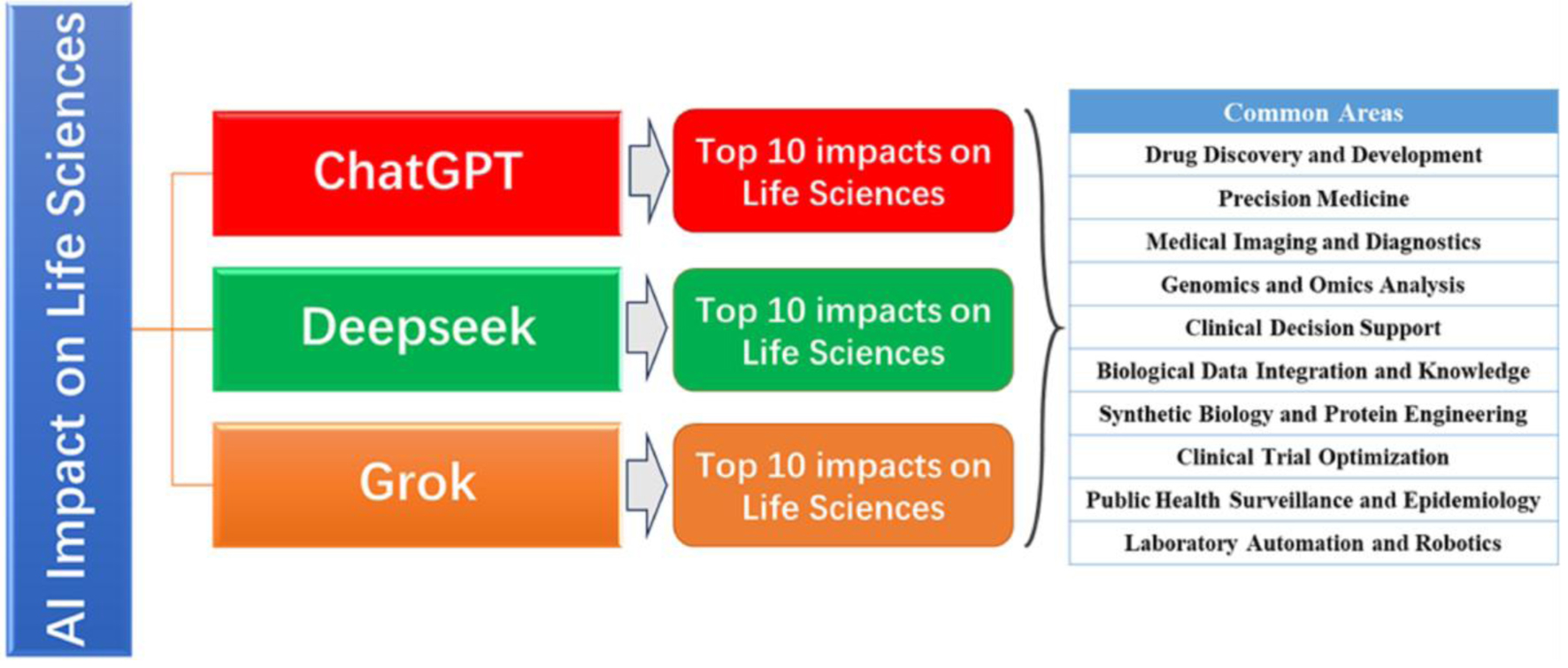 Click for large image | Figure 1. Top 10 impacts of artificial intelligence (AI) on life sciences. This figure illustrates the key domains where AI technologies are transforming life sciences research and application. We highlight the most common intersections among ChatGPT, DeepSeek, and Grok, ranked by frequency and relevance. From accelerating drug discovery and development to enabling precision medicine, AI enhances decision-making by integrating vast and complex biomedical data. Medical imaging and diagnostics benefit from AI-powered image recognition, while genomics and omics analysis leverage machine learning to interpret large-scale sequencing data. Clinical decision support systems use AI to improve patient outcomes, and biological data integration facilitates knowledge extraction from heterogeneous sources. In synthetic biology and protein engineering, AI aids in designing novel biomolecules and functions. Clinical trial optimization is advanced through predictive analytics, improving recruitment and success rates. AI also plays a crucial role in public health surveillance, modeling disease spread and guiding interventions. Finally, laboratory automation and robotics streamline experimental workflows, enabling high-throughput and reproducible research. Collectively, these domains highlight the profound and multidisciplinary influence of AI on modern life sciences. |
 Click to view | Table 1. Top 10 Impacts That AI May Have on Life Sciences Identified by ChatGPT, DeepSeek and Grok |
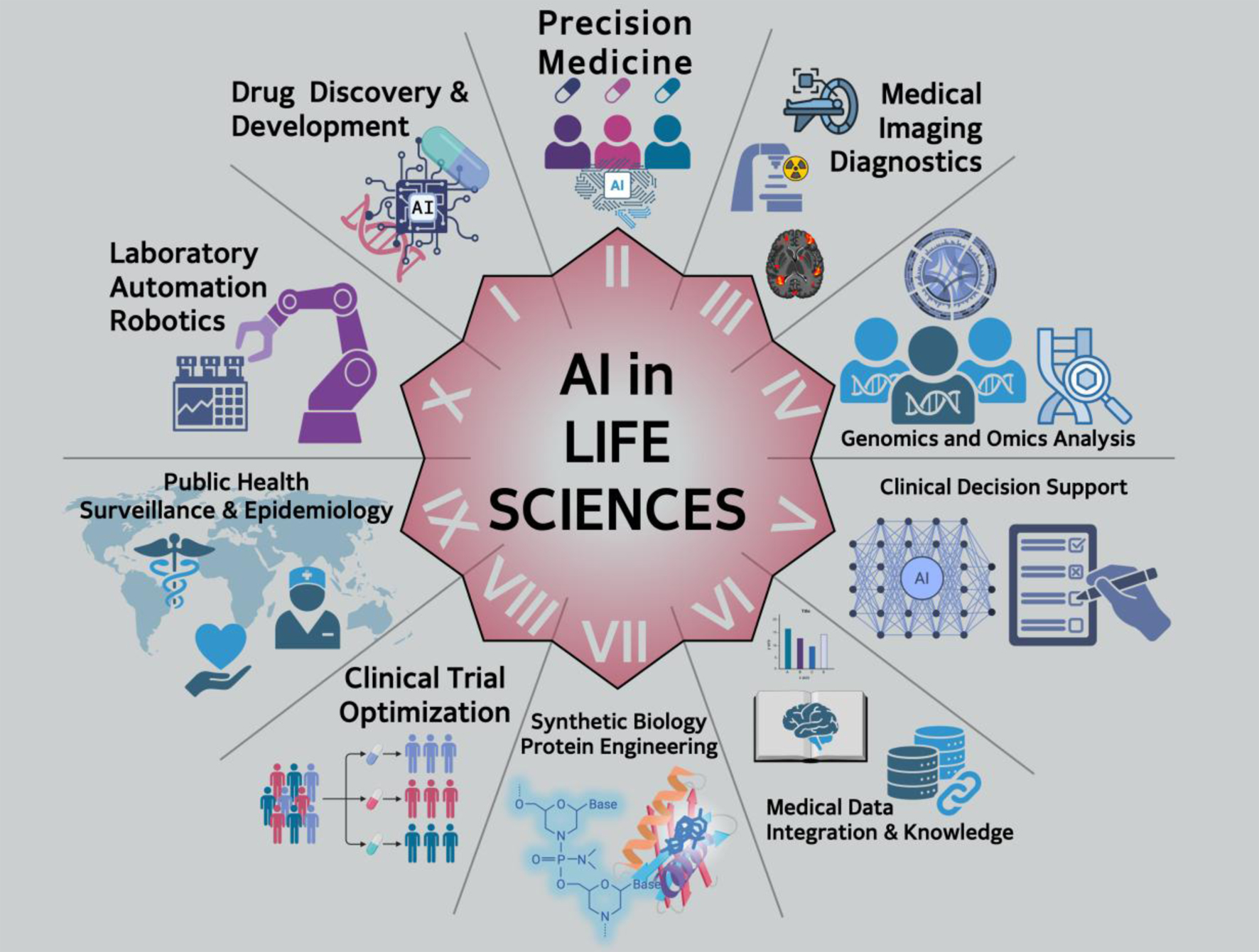 Click for large image | Figure 2. The top 10 impacts of artificial intelligence (AI) on life sciences. This diagram highlights 10 key domains where AI is transforming life sciences. AI is accelerating drug discovery and development by predicting molecular activity and optimizing compound screening. In precision medicine, it integrates genomic, clinical, and lifestyle data to tailor therapies. AI enhances medical imaging and diagnostics through advanced pattern recognition, improving detection of diseases from radiological and histological data. In genomics and omics analysis, machine learning deciphers complex datasets to identify disease mechanisms. Clinical decision support systems use AI to improve diagnosis and treatment recommendations. AI facilitates biological data integration and knowledge extraction by synthesizing insights across diverse sources. In synthetic biology and protein engineering, it enables design and modeling of novel biomolecules. AI improves clinical trial optimization by enhancing recruitment, predicting outcomes, and reducing costs. Public health surveillance and epidemiology benefit from AI’s predictive capabilities for disease outbreaks. Finally, laboratory automation and robotics streamline workflows and increase research productivity. |
| Drug Discovery and Development | ▴Top |
AI is revolutionizing drug discovery and development by streamlining and accelerating processes that traditionally required years of work and immense financial investment. AI can efficiently predict molecule-target interactions using deep learning models trained on chemical structures, protein databases, and biological activity data. These models help identify potential drug candidates by forecasting binding affinities, off-target effects, and pharmacokinetic properties [7].
One major application is de novo drug design, where AI algorithms generate novel molecular structures optimized for specific biological targets. Generative models such as variational autoencoders (VAEs), generative adversarial networks (GANs), and reinforcement learning frameworks are used to create compounds with desirable drug-like properties [5]. These methods significantly reduce the time and cost needed for initial lead discovery and optimization.
AI also plays a pivotal role in clinical trial design and recruitment. ML models can analyze real-world data (e.g., EHRs, multi-omics data) to identify patient subgroups most likely to respond to a therapy, thus enhancing trial efficiency and reducing attrition [3, 4].
Perhaps the most widely celebrated breakthrough in this space is AlphaFold, developed by DeepMind. In 2020, AlphaFold demonstrated an unprecedented ability to accurately predict protein 3D structures from amino acid sequences, solving a 50-year grand challenge in biology. This innovation enables researchers to better understand disease mechanisms and accelerate the identification of druggable targets [1, 8]. The open release of AlphaFold-predicted structures for the human proteome has already catalyzed new research and drug development programs across academia and industry.
| Precision Medicine | ▴Top |
AI is a driving force behind the advancement of precision medicine, where treatment strategies are tailored to the individual characteristics of each patient. This approach integrates diverse data types, such as genomic, transcriptomic, proteomic, metabolomic, and EHR data, using advanced ML models to predict disease risk, treatment responses, and optimal interventions.
AI algorithms excel at analyzing large, multidimensional datasets, uncovering complex patterns that are not apparent through conventional methods. For instance, deep learning models can identify gene expression signatures associated with therapy response or resistance, enabling personalized treatment decisions [9]. In oncology, AI models have been used to match patients to targeted therapies based on tumor mutation profiles, leading to better clinical outcomes [10].
EHRs, which contain longitudinal data on patient history, medications, and lab results, can be mined using NLP and ML to generate predictive risk models. These models support clinical decision-making in areas such as cancer care, cardiology, and diabetes management [11]. In one example, the PREDICT algorithm, developed by the UK’s National Health Service (NHS), uses patient-specific tumor and demographic data to guide breast cancer treatment decisions, improving both survival outcomes and quality of life [12].
The integration of AI into tumor profiling has been transformative. Tools like IBM Watson for Genomics interpret sequencing data to suggest personalized cancer treatment options, drawing on curated literature, clinical guidelines, and trial databases [13].
| Medical Imaging and Diagnostics | ▴Top |
AI is significantly enhancing the accuracy, speed, and consistency of medical imaging interpretation across radiology and pathology, two of the most data-intensive fields in healthcare. By leveraging deep learning, especially convolutional neural networks (CNNs), AI systems can process complex image data to detect patterns that may escape human observers, offering powerful decision-support tools for clinical diagnosis.
In radiology, AI models have been trained to analyze computed tomography (CT) scans, magnetic resonance imaging (MRI), and X-rays to detect abnormalities such as lung nodules, fractures, brain hemorrhages, and signs of pneumonia. One landmark study by McKinney et al demonstrated that a deep learning system developed by Google Health outperformed human radiologists in detecting breast cancer on mammograms [14]. Similarly, AI has shown promise in identifying lung cancer from low-dose CT scans, with performance comparable to or exceeding that of expert radiologists [15].
In digital pathology, AI is being used to analyze high-resolution histopathological slides for cancer grading, biomarker quantification, and detection of rare cellular events. For example, AI systems have demonstrated expert-level performance in diagnosing prostate cancer from biopsy slides and in detecting lymph node metastases in breast cancer [16]. These tools offer high-throughput, reproducible assessments that assist pathologists in making more accurate diagnoses.
Importantly, AI can also standardize interpretations, minimize diagnostic variability, and improve access to expert-level diagnostics in underserved or remote areas. As regulatory approvals increase, AI-powered diagnostic tools are being integrated into clinical workflows worldwide, enhancing both diagnostic precision and efficiency.
| Genomics and Omics Analysis | ▴Top |
AI plays a critical role in unlocking insights from the high-dimensional and complex datasets generated by modern omics technologies - genomics, transcriptomics, proteomics, and metabolomics. These data layers are essential for understanding the molecular basis of disease, identifying therapeutic targets, and developing personalized treatments.
In genomics, AI algorithms accelerate the detection and annotation of genetic variants from whole-genome or exome sequencing data. Traditional pipelines rely heavily on rule-based filters and manual curation, but AI-based tools can prioritize functional and disease-relevant mutations with greater accuracy. Deep learning models like DeepVariant, developed by Google, have been shown to outperform conventional methods in identifying single nucleotide variants and insertions/deletions from sequencing data [17].
In transcriptomics, ML helps uncover hidden gene expression patterns, regulatory modules, and disease subtypes. For example, unsupervised learning techniques such as clustering and dimensionality reduction (e.g., t-distributed stochastic neighbor embedding (t-SNE), uniform manifold approximation and projection (UMAP)) are used to analyze single-cell RNA-seq data, revealing cellular heterogeneity and lineage trajectories in cancer, immune responses, and development [18].
Proteomics and metabolomics also benefit from AI in spectral data analysis, biomarker discovery, and pathway modeling. AI methods enable the integration of multi-omics data, offering a holistic view of biological systems and revealing novel disease mechanisms. Multi-modal ML approaches are especially powerful in identifying cross-talk between genomic alterations and downstream protein or metabolite changes [19].
The ultimate impact of AI in omics is its ability to translate massive datasets into actionable insights, from identifying cancer-driving mutations to uncovering metabolic signatures of neurodegenerative diseases, thus transforming both research and clinical practice.
| Clinical Decision Support | ▴Top |
AI-powered Clinical Decision Support Systems (CDSS) are transforming how clinicians interpret patient data and make diagnostic and treatment decisions. These tools combine ML, NLP, and real-time data analytics to recommend diagnoses, predict risks, or suggest treatment strategies based on current evidence and patient-specific characteristics.
One major advantage of AI in CDSS is its ability to synthesize complex and large-scale data, including EHRs, imaging, genomics, and biomedical literature. AI models can flag potential diagnostic errors, suggest differential diagnoses, predict disease progression, and alert clinicians to adverse drug interactions, all of which enhance patient safety and reduce cognitive burden [20].
Early applications like IBM Watson for Oncology showcased the potential of AI to match patients with evidence-based cancer treatments. By integrating clinical guidelines, medical literature, and patient data, Watson aimed to recommend personalized treatment options. In a study from India, Watson’s treatment recommendations aligned with oncologists’ decisions in over 90% of breast cancer cases, demonstrating clinical concordance [21].
Beyond oncology, AI-CDSS tools have been successfully implemented for sepsis detection, heart failure prediction, and triage support in emergency medicine. For example, DeepMind developed an AI system capable of predicting acute kidney injury (AKI) up to 48 h before onset, enabling earlier intervention and improved outcomes [22].
Despite these promising results, the integration of AI into clinical workflows requires rigorous validation, clinician oversight, and transparency in algorithmic decision-making. When properly implemented, AI-CDSS can significantly improve diagnostic accuracy, reduce medical errors, and contribute to more consistent, equitable care.
| Biological Data Integration and KGs | ▴Top |
AI plays a pivotal role in integrating heterogeneous biomedical data sources, including scientific literature, omics datasets, drug databases, and clinical trial results, into structured knowledge networks such as KGs. These AI-driven frameworks enable researchers to uncover novel relationships between genes, diseases, drugs, and phenotypes, facilitating systems-level understanding of biology and accelerating hypothesis generation.
KGs organize information as nodes (e.g., genes, proteins, diseases, drugs) and edges (relationships between them), enabling semantic reasoning and pattern discovery. AI techniques such as NLP and graph neural networks (GNNs) help automate KG construction and inference from unstructured text (e.g., PubMed abstracts) and structured databases (e.g., DrugBank, OMIM). For instance, ML over literature-derived KGs proposes previously unknown disease-gene associations and drug repurposing opportunities [23, 24].
In systems biology, integrating omics data with KGs allows dynamic modeling of pathways and molecular networks, such as those involved in cancer progression or immune regulation. These models support biomarker discovery, drug target prioritization, and prediction of combinatorial therapies [25]. For example, Hetionet, an integrative KG combining 29 public biomedical resources, has been used to systematically predict new drug-disease relationships [26].
AI-powered data integration also enhances the interpretability of high-throughput experiments by connecting genes or proteins of interest to known biological processes, phenotypes, or clinical outcomes, significantly improving research productivity and translational relevance.
| Synthetic Biology and Protein Engineering | ▴Top |
AI is rapidly advancing synthetic biology and protein engineering by enabling the rational design of biological molecules such as enzymes, therapeutic proteins, and synthetic genetic circuits. Traditionally, protein engineering relied on trial-and-error mutagenesis and labor-intensive screening. Today, AI, especially deep learning, facilitates the prediction of structure-function relationships, de novo protein design, and synthetic pathway optimization with unprecedented precision.
Generative models, including VAEs, GANs, and transformers, can create novel protein sequences tailored for specific functions. Tools such as ProtGPT2 and ESMFold, trained on massive protein databases, can suggest mutations that enhance stability, binding, or catalytic efficiency [27, 28].
AI is also transforming enzyme design. Deep learning models can predict active site architecture, substrate specificity, and evolutionary fitness, accelerating the discovery of enzymes for applications in biocatalysis, biofuels, and environmental remediation. For example, ML has been used to engineer more efficient PETase enzymes for degrading plastic waste [29].
In metabolic engineering, AI guides the optimization of microbial biosynthetic pathways by identifying genetic modifications that improve yield, minimize byproducts, and balance flux in organisms such as E. coli and S. cerevisiae. Reinforcement learning frameworks enable iterative optimization of synthetic pathways in silico before lab implementation [30].
Furthermore, AI contributes to CRISPR guide RNA design by improving on-target efficiency and minimizing off-target effects. Predictive tools like DeepCRISPR and CRISPR-Net support customized genome editing strategies for therapeutic applications.
| Clinical Trial Optimization | ▴Top |
AI is increasingly used to optimize clinical trials, addressing long-standing challenges such as patient recruitment, trial design, and outcome prediction. By analyzing vast volumes of real-world data, particularly from EHRs, imaging, genomic profiles, and claims data, AI supports more efficient, precise, and cost-effective clinical trial execution.
One of the major barriers to trial success is patient recruitment, with over 80% of trials failing to meet enrollment targets. AI algorithms can mine EHRs to automatically identify patients who meet complex inclusion and exclusion criteria, significantly accelerating recruitment timelines. For example, deep learning systems can extract both structured and unstructured data, such as diagnosis codes, lab values, and clinical notes, to screen candidates at scale [31].
AI also improves patient stratification by identifying subgroups more likely to respond to a given therapy, enabling adaptive trial designs and personalized interventions. In oncology, predictive models that combine genomic and phenotypic data help select high-responder populations, increasing trial efficiency and statistical power [32].
Another important application is dropout risk prediction. ML models trained on demographic, behavioral, and clinical variables can flag participants at risk of non-adherence or attrition, allowing for early intervention and improved retention [33].
AI tools are also used to simulate virtual control arms, optimize endpoint selection, and enable real-time trial monitoring. These capabilities reduce trial duration and costs. Major pharmaceutical companies such as Pfizer, Novartis, and Roche are integrating AI across the clinical trial pipeline to accelerate regulatory approvals and reduce development risks.
| Public Health Surveillance and Epidemiology | ▴Top |
AI has become a powerful tool in public health surveillance and epidemiology, offering real-time, data-driven insights for tracking disease outbreaks, predicting epidemic trends, and guiding public health policy. Traditional surveillance methods often suffer from latency and limited granularity, while AI enables faster, more scalable, and more accurate detection and forecasting of disease spread.
One landmark example is BlueDot, a Canadian company that uses NLP and ML to scan over 100,000 global data sources daily, including news articles, airline data, and health reports. BlueDot detected early signs of the coronavirus disease 2019 (COVID-19) outbreak in Wuhan on December 31, 2019, days before the World Health Organization issued a public alert [34].
AI is also essential in pandemic modeling, helping simulate disease transmission dynamics and evaluate the impact of public health interventions. For instance, deep learning models combined with compartmental approaches (e.g., susceptible, exposed, infectious, and recovered (SEIR) models) were used to predict COVID-19 cases, hospital loads, and the effectiveness of lockdowns and vaccination campaigns [35].
In addition, AI tools mine social media, mobility patterns, and search engine trends to identify early signs of emerging health threats. Platforms like HealthMap and the now-retired Google Flu Trends exemplify this approach, using digital footprints as proxies for real-world epidemiological trends.
Beyond infectious disease, AI is increasingly applied to chronic disease epidemiology, environmental exposure analysis, and the study of social determinants of health, supporting more proactive and predictive public health strategies.
| Laboratory Automation and Robotics | ▴Top |
AI-powered laboratory automation and robotics are transforming the pace and precision of biomedical research. These intelligent systems streamline repetitive and time-consuming tasks, such as pipetting, plating, imaging, and data logging, while enabling real-time decision-making and adaptive experimentation based on live feedback from data.
In high-throughput screening (HTS), AI-driven robots can handle thousands of experimental conditions simultaneously, analyzing chemical libraries or genetic perturbations at a scale unmanageable by humans. These platforms incorporate computer vision, sensor technologies, and ML to identify patterns in assay outputs, reducing false positives and optimizing experimental conditions [36].
One groundbreaking example is the “robot scientist” Eve, which uses AI to automate the design, execution, and analysis of drug discovery experiments. Eve was able to independently identify a potential antimalarial compound by screening and analyzing a library of existing drugs, demonstrating how autonomous labs can generate meaningful biological discoveries [37].
AI also plays a key role in adaptive laboratory evolution (ALE), where microbial strains or proteins are evolved for improved traits. Algorithms guide the selection of conditions or mutations based on predicted performance, significantly accelerating evolution cycles [38].
Furthermore, real-time AI analytics are integrated into platforms like Lab-on-a-Chip systems and automated microscopy, allowing for instant feedback loops where experimental parameters are adjusted on the fly to maximize data quality and biological relevance. These technologies boost reproducibility and scalability, addressing major challenges in biomedical research reproducibility [39].
As AI-integrated robotics become more accessible, they are democratizing access to intelligent laboratories, allowing researchers to run complex experiments with minimal manual intervention, transforming productivity across academia, biotech, and pharma [40].
| Conclusion | ▴Top |
AI is revolutionizing life sciences by accelerating discoveries and improving healthcare outcomes. In drug development, it enables faster therapeutic candidate identification and optimized clinical trials. Precision medicine leverages AI to analyze complex patient data for personalized treatments. Diagnostic tools achieve expert-level accuracy in medical imaging and pathology, while multi-omics analysis uncovers novel disease mechanisms. AI-powered clinical decision systems enhance diagnosis and treatment planning. KGs integrate diverse biological data, revealing new therapeutic insights. Synthetic biology benefits from AI-designed proteins and enzymes, and clinical trials become more efficient through improved patient selection and retention. Public health systems utilize AI for early outbreak detection and pandemic modeling. Laboratory automation, enhanced by AI, increases research reproducibility and throughput. Together, these applications demonstrate AI’s transformative potential across the entire biomedical spectrum, from fundamental research to clinical implementation, ushering in a new era of data-driven, efficient, and personalized life sciences innovation. Continued advancements promise even greater impacts as AI technologies mature and integrate deeper into scientific workflows.
Acknowledgments
We would like to express our sincere gratitude to Dr. Licun Wu for inviting us to contribute our manuscript to this innovative journal. His overarching vision and encouragement were instrumental in the development and completion of this article. We also thank Dr. Kui Chen for his valuable contribution in creating the diagram presented in Figure 2.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest to disclose.
Author Contributions
Geng Zang and Zilong Wang conceptualized and designed the study. They both conducted the literature review, analyzed the scientific advancements across the 10 AI application domains, and synthesized the findings into a cohesive manuscript. They also used AI platforms to draft and revise the manuscript for intellectual content. Both authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583-589.
doi pubmed - Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts H. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18(8):500-510.
doi pubmed - Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56.
doi pubmed - Zhavoronkov A, Ivanenkov YA, Aliper A, Veselov MS, Aladinskiy VA, Aladinskaya AV, Terentiev VA, et al. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat Biotechnol. 2019;37(9):1038-1040.
doi pubmed - Gomez-Bombarelli R, Wei JN, Duvenaud D, Hernandez-Lobato JM, Sanchez-Lengeling B, Sheberla D, Aguilera-Iparraguirre J, et al. Automatic chemical design using a data-driven continuous representation of molecules. ACS Cent Sci. 2018;4(2):268-276.
doi pubmed - Han X, Zhang S, Zhou DC, Wang D, He X, Yuan D, Li R, et al. MSIsensor-ct: microsatellite instability detection using cfDNA sequencing data. Brief Bioinform. 2021;22(5):bbaa402.
doi pubmed - Chen H, Engkvist O, Wang Y, Olivecrona M, Blaschke T. The rise of deep learning in drug discovery. Drug Discov Today. 2018;23(6):1241-1250.
doi pubmed - Wallach I, Dzamba M, Heifets A. AtomNet: A deep convolutional neural network for bioactivity prediction in structure-based drug discovery. arXiv [cs.LG]. 2015.
- Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8-17.
doi pubmed - Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20(2):273-286.
doi pubmed - Wang RH, Wang ZL, Song ZY, Buckeridge D, Li Y. MixEHR-nest: identifying subphenotypes within electronic health records through hierarchical guided-topic modeling. In Proc. 15th ACM Conf. Bioinformatics, Comput. Biol. Health Informatics (BCB’ 24). Article 53. 2024; p. 1-8.
- Wishart GC, Azzato EM, Greenberg DC, Rashbass J, Kearins O, Lawrence G, Caldas C, et al. PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res. 2010;12(1):R1.
doi pubmed - Lee WS, Ahn SM, Chung JW, Kim KO, Kwon KA, Kim Y, Sym S, et al. Assessing concordance with Watson for oncology, a cognitive computing decision support system for colon cancer treatment in Korea. JCO Clin Cancer Inform. 2018;2:1-8.
doi pubmed - McKinney SM, Sieniek M, Godbole V, Godwin J, Antropova N, Ashrafian H, Back T, et al. International evaluation of an AI system for breast cancer screening. Nature. 2020;577(7788):89-94.
doi pubmed - Ardila D, Kiraly AP, Bharadwaj S, Choi B, Reicher JJ, Peng L, Tse D, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25(6):954-961.
doi pubmed - Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, van der Laak J, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210.
doi pubmed - Poplin R, Chang PC, Alexander D, Schwartz S, Colthurst T, Ku A, Newburger D, et al. A universal SNP and small-indel variant caller using deep neural networks. Nat Biotechnol. 2018;36(10):983-987.
doi pubmed - Kiselev VY, Andrews TS, Hemberg M. Challenges in unsupervised clustering of single-cell RNA-seq data. Nat Rev Genet. 2019;20(5):273-282.
doi pubmed - Zitnik M, Nguyen F, Wang B, Leskovec J, Goldenberg A, Hoffman MM. Machine learning for integrating data in biology and medicine: principles, practice, and opportunities. Inf Fusion. 2019;50:71-91.
doi pubmed - Sendak MP, et al. A path for translation of machine learning products into healthcare delivery. NPJ Digit Med. 2020;3:1-10.
- Kim EJ, Woo HS, Cho JH, Sym SJ, Baek JH, Lee WS, Kwon KA, et al. Early experience with Watson for oncology in Korean patients with colorectal cancer. PLoS One. 2019;14(3):e0213640.
doi pubmed - Tomasev N, Glorot X, Rae JW, Zielinski M, Askham H, Saraiva A, Mottram A, et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature. 2019;572(7767):116-119.
doi pubmed - Zitnik M, Agrawal M, Leskovec J. Modeling polypharmacy side effects with graph convolutional networks. Bioinformatics. 2018;34(13):i457-i466.
doi pubmed - Foster N, et al. IBM Watson AI-enhanced search tool identifies novel candidate genes and provides insight into potential pathomechanisms of traumatic heterotopic ossification. Burns Open. 2023;7(4):126-138.
- Spangler S. et al. Automated hypothesis generation based on mining scientific literature. Proc. 20th ACM SIGKDD Int. Conf. Knowl. Discov. Data Min. 2014:1877-1886.
- Himmelstein DS, Lizee A, Hessler C, Brueggeman L, Chen SL, Hadley D, Green A, et al. Systematic integration of biomedical knowledge prioritizes drugs for repurposing. Elife. 2017;6.
doi pubmed - Madani A, Krause B, Greene ER, Subramanian S, Mohr BP, Holton JM, Olmos JL, Jr., et al. Large language models generate functional protein sequences across diverse families. Nat Biotechnol. 2023;41(8):1099-1106.
doi pubmed - Rives A, Meier J, Sercu T, Goyal S, Lin Z, Liu J, Guo D, et al. Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Proc Natl Acad Sci U S A. 2021;118(15):e2016239118.
doi pubmed - Lu H, et al. Machine learning-aided engineering of hydrolases for PET degradation. Nature Catalysis. 2022;5(8):673-681.
- Zampieri G, Vijayakumar S, Yaneske E, Angione C. Machine and deep learning meet genome-scale metabolic modeling. PLoS Comput Biol. 2019;15(7):e1007084.
doi pubmed - Weng C, Li Y, Ryan P, Zhang Y, Liu F, Gao J, Bigger JT, et al. A distribution-based method for assessing the differences between clinical trial target populations and patient populations in electronic health records. Appl Clin Inform. 2014;5(2):463-479.
doi pubmed - Liu R, Rizzo S, Whipple S, Pal N, Pineda AL, Lu M, Arnieri B, et al. Evaluating eligibility criteria of oncology trials using real-world data and AI. Nature. 2021;592(7855):629-633.
doi pubmed - Kavalci E, Hartshorn A. Improving clinical trial design using interpretable machine learning based prediction of early trial termination. Sci Rep. 2023;13(1):121.
doi pubmed - Bogoch, II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. 2020;27(2):taaa008.
doi pubmed - Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, Pastore YPA, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395-400.
doi pubmed - Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, Green DV, et al. Impact of high-throughput screening in biomedical research. Nat Rev Drug Discov. 2011;10(3):188-195.
doi pubmed - King RD, Rowland J, Oliver SG, Young M, Aubrey W, Byrne E, Liakata M, et al. The automation of science. Science. 2009;324(5923):85-89.
doi pubmed - Borkowski O, Koch M, Zettor A, Pandi A, Batista AC, Soudier P, Faulon JL. Large scale active-learning-guided exploration for in vitro protein production optimization. Nat Commun. 2020;11(1):1872.
doi pubmed - Ha T, Lee D, Kwon Y, Park MS, Lee S, Jang J, Choi B, et al. AI-driven robotic chemist for autonomous synthesis of organic molecules. Sci Adv. 2023;9(44):eadj0461.
doi pubmed - Gao S, Fang A, Huang Y, Giunchiglia V, Noori A, Schwarz JR, Ektefaie Y, et al. Empowering biomedical discovery with AI agents. Cell. 2024;187(22):6125-6151.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
AI in Clinical Medicine is published by Elmer Press Inc.