| AI in Clinical Medicine, ISSN 0000-0000 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, AI Clin Med and Elmer Press Inc |
| Journal website https://aicm.elmerpub.com |
Review
Volume 1, 2025, e9
Benefits of AI in Transforming Cancer Care
Nan Wu
Sunnybrook Health Sciences Centre, Toronto, Canada
Manuscript submitted September 4, 2025, accepted September 12, 2025, published online September 18, 2025
Short title: Benefits of AI in Transforming Cancer Care
doi: https://doi.org/10.14740/aicm9
- Abstract
- Introduction
- Earlier and More Accurate Diagnosis
- Personalized Treatment Planning
- Drug Discovery and Access to Novel Therapies
- Optimizing Radiotherapy and Surgery
- Continuous Monitoring and Early Recurrence Detection
- Enhanced Supportive and Palliative Care
- Health Equity and Access
- References
| Abstract | ▴Top |
Artificial intelligence (AI) is rapidly emerging as a transformative force in oncology, offering significant benefits across the cancer care continuum. Through advanced image analysis, AI enables earlier and more accurate diagnosis by detecting subtle abnormalities in radiology, pathology, and liquid biopsy data that may elude conventional assessment. Integrating multi-omics, clinical, and imaging datasets, AI supports highly personalized treatment planning, predicting therapeutic responses and guiding the selection of targeted agents and immunotherapies. Machine learning models also facilitate rapid drug discovery and repurposing, and improve patient access to clinical trials by matching tumor molecular profiles with trial eligibility criteria. In local therapies, AI enhances surgical navigation and radiotherapy planning, increasing precision while sparing healthy tissues. Continuous patient monitoring through wearable devices, electronic health records, and laboratory data allows AI systems to identify complications or recurrence earlier than standard follow-up methods. In supportive and palliative care, AI-driven tools anticipate side effects, optimize symptom management, and provide language, literacy, and psychological support. Furthermore, AI-enabled tele-oncology and translation services expand cancer care to underserved populations, addressing disparities in access. While ethical, regulatory, and technical challenges remain, the integration of AI into oncology holds immense promise for improving diagnostic accuracy, therapeutic efficacy, and quality of life for cancer patients worldwide.
Keywords: Artificial intelligence; Oncology; Early cancer diagnosis; Precision medicine; Clinical decision support; Radiotherapy optimization; Tele-oncology; Cancer health equity
| Introduction | ▴Top |
The launch of ChatGPT [1] in November 2022 marked the beginning of a transformative era in healthcare artificial intelligence (AI), with platforms like DeepSeek [2], Grok [3], and Perplexity [4] rapidly advancing to revolutionize cancer care. These sophisticated tools are enhancing oncology through improved diagnostic accuracy, personalized treatment planning, and predictive analytics while accelerating drug discovery and providing virtual patient support. By streamlining clinical workflows and democratizing access to expert knowledge, these AI systems serve not as replacements for physicians but as powerful augmentations to their capabilities, enabling more precise, efficient, and patient-centered care. As this technology continues to evolve, its integration into cancer treatment promises to deliver better survival outcomes, reduce healthcare costs, and significantly improve quality of life for patients globally, ushering in a new standard of oncological practice that combines cutting-edge AI with human expertise.
AI is rapidly transforming oncology, offering new opportunities to improve prevention, diagnosis, treatment, and survivorship. Advances in machine learning (ML), deep learning (DL), and natural language processing (NLP) now enable integration of multimodal datasets—including medical imaging, histopathology, genomics, and electronic health records (EHRs)—into clinically actionable insights.
In diagnostics, AI systems have achieved radiologist-level or superior performance in detecting breast, lung, and skin cancers, often identifying subtle features imperceptible to the human eye, thereby enabling earlier intervention and improved patient outcomes [5, 6]. In pathology, AI-driven whole-slide image analysis can quantify histopathological features, detect rare events, and even predict molecular subtypes to guide targeted therapies [7].
For treatment personalization, predictive AI models integrate molecular and clinical data to forecast individual responses to chemotherapy, immunotherapy, and radiotherapy, optimizing efficacy while minimizing toxicity [8, 9]. In drug discovery, AI accelerates target identification, compound optimization, and clinical trial design, shortening timelines from years to months [10].
AI also enables real-time monitoring through wearables, digital symptom trackers, and liquid biopsies, allowing early detection of recurrence and rapid intervention [11]. AI-powered virtual assistants enhance patient engagement, improve health literacy, and extend oncology expertise to underserved regions [12].
AI is redefining oncology by streamlining clinical workflows, minimizing diagnostic errors, and democratizing access to precision medicine. As AI integration in healthcare deepens, its synergy with physician expertise demonstrates significant potential to improve patient survival rates, reduce costs, and enhance quality of life globally. Our comprehensive analysis of leading AI platforms (ChatGPT, DeepSeek, Grok, and Perplexity) identified seven key transformative benefits for cancer care, categorized across four domains: diagnostics, treatment, research, and patient support (Tables 1-4). These findings demonstrate how AI is revolutionizing oncology through earlier detection, personalized therapy, accelerated drug development, and enhanced patient outcomes (Table 5, Fig. 1).
 Click to view | Table 1. ChatGPT: Concise List of Key Benefits AI Brings to Cancer Care |
 Click to view | Table 2. DeepSeek: Concise List of Key Benefits AI Brings to Cancer Care |
 Click to view | Table 3. Grok: Concise List of Key Benefits AI Brings to Cancer Care |
 Click to view | Table 4. Perplexity: Concise List of Key Benefits AI Brings to Cancer Care |
 Click to view | Table 5. Cross-Platform Comparison of AI Capabilities in Cancer Care |
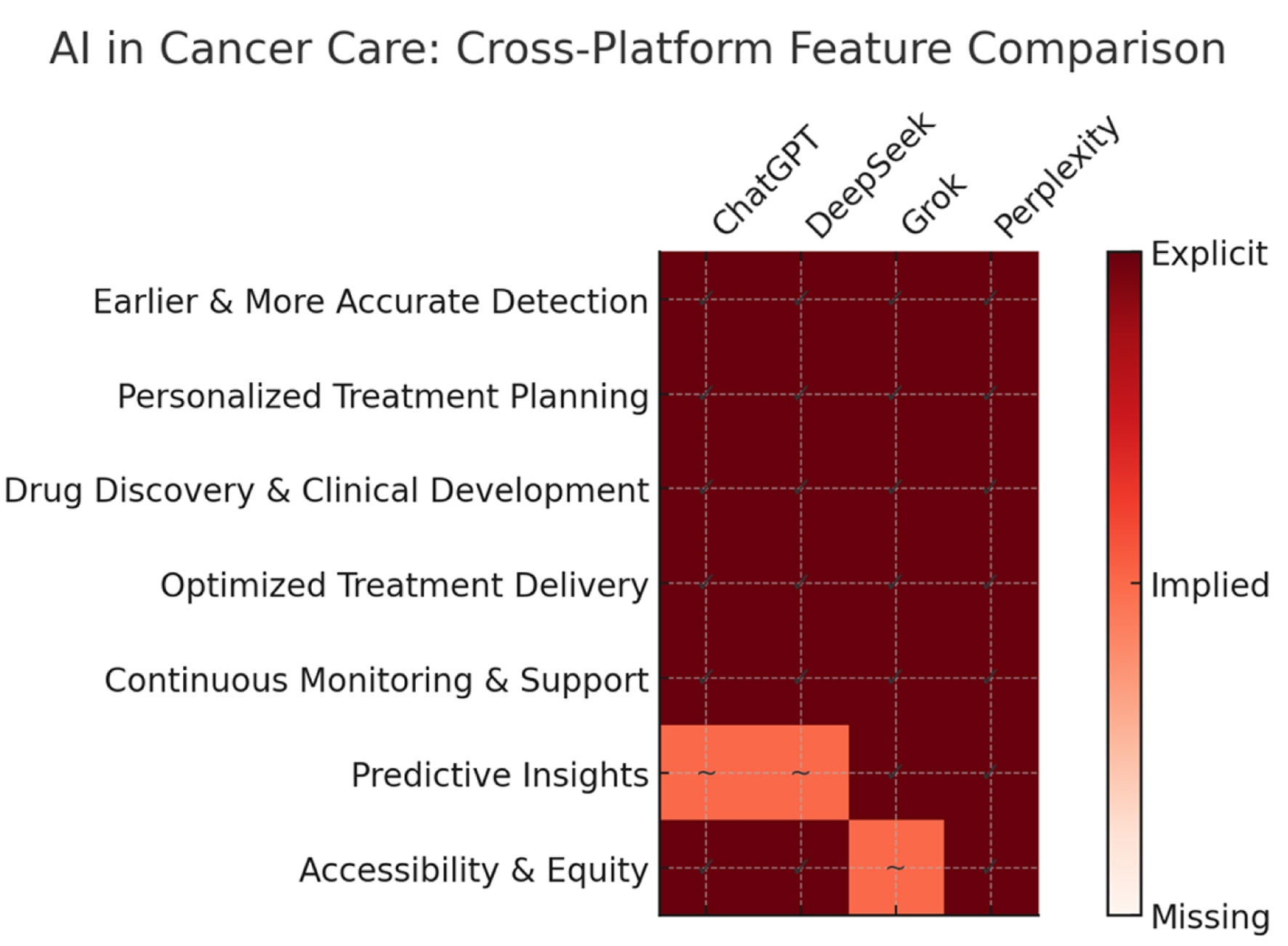 Click for large image | Figure 1. Comparative analysis of AI platforms in cancer care. This schematic diagram compares seven key AI-enabled benefits in cancer care across four platforms: ChatGPT, DeepSeek, Grok, and Perplexity. Rows represent unified benefit categories, and columns represent platforms. Color intensity reflects coverage: dark red with “✓” indicates explicitly mentioned benefits, light red with “∼” indicates benefits implied but not explicitly stated, and white indicates benefits not covered. The chart highlights strong consensus across platforms in early detection, personalization, drug discovery, optimized treatment delivery, and monitoring. Variability is most evident in predictive analytics and accessibility/equity, where some platforms show implied rather than explicit coverage. This visual enables quick cross-platform alignment assessment. |
| Earlier and More Accurate Diagnosis | ▴Top |
Early cancer detection is critical to improving survival outcomes, and AI is playing a pivotal role in enhancing diagnostic accuracy across imaging, pathology, and liquid biopsy platforms.
In medical imaging, DL-based algorithms can detect malignancies in computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and mammographic scans with sensitivity and specificity that match or exceed expert radiologists. These models excel at identifying subtle or subclinical disease features that are often imperceptible to the human eye, thereby enabling diagnosis at earlier stages and facilitating timely intervention [5, 6]. For example, convolutional neural networks have demonstrated high performance in breast cancer screening, reducing both false negatives and false positives, while AI models for low-dose CT scans have improved early lung cancer detection rates [6].
In digital pathology, AI systems can process gigapixel whole-slide images to identify cancer subtypes, quantify mitotic rates, and detect clinically relevant biomarkers with high precision [7, 13]. Weakly supervised learning approaches allow these algorithms to operate effectively even with limited annotation, while multi-task networks can integrate histopathological and molecular data to inform treatment planning. Such tools can also flag diagnostically challenging or rare cases for secondary review, reducing the risk of oversight.
In liquid biopsy interpretation, AI enhances the analysis of circulating tumor DNA (ctDNA), exosomes, and other blood-based biomarkers to detect minimal residual disease or emerging recurrence before radiographic evidence [11, 14]. By integrating sequencing data with clinical variables, ML models can distinguish cancer-derived signals from background noise, improving both sensitivity and specificity.
Collectively, these AI-enabled diagnostic tools promise to shift cancer detection toward earlier stages, when curative treatment is more likely, ultimately improving survival rates and quality of life for patients worldwide.
| Personalized Treatment Planning | ▴Top |
Personalized cancer therapy aims to tailor interventions to the unique molecular, clinical, and lifestyle characteristics of each patient. AI is increasingly central to this approach, integrating complex datasets to guide precise treatment choices, forecast therapeutic outcomes, and adapt regimens in real time.
In genomic profiling integration, AI can analyze vast next-generation sequencing (NGS) datasets to identify actionable mutations, gene fusions, and copy number variations, enabling precise matching of patients to targeted therapies or immunotherapies [8, 15]. By incorporating data from The Cancer Genome Atlas (TCGA) and other large-scale repositories, AI platforms can also detect rare or novel driver alterations, expanding the range of potential treatment options [16].
Predictive models use ML to anticipate treatment responses by integrating tumor molecular features, histopathological parameters, comorbidities, and prior treatment outcomes from similar patients [9, 17]. These models can help oncologists select regimens with the highest probability of efficacy while minimizing adverse events. For example, DL-based radiomics combined with genomics can predict immunotherapy benefit in non-small cell lung cancer (NSCLC), allowing for more targeted patient selection [18].
Adaptive therapy applies AI’s continuous learning capabilities to modify treatment plans as the patient’s tumor evolves under therapeutic pressure. By integrating longitudinal imaging, ctDNA profiles, and clinical biomarkers, AI systems can detect early resistance and suggest timely regimen adjustments [11, 19]. This dynamic approach helps prolong treatment efficacy, delay disease progression, and avoid unnecessary toxicity.
Together, these AI-powered strategies enable a shift from one-size-fits-all protocols toward data-driven, precision oncology, ultimately improving treatment outcomes, quality of life, and cost-effectiveness in cancer care.
| Drug Discovery and Access to Novel Therapies | ▴Top |
Drug discovery in oncology has traditionally been a lengthy, high-cost process, often taking over a decade from target identification to regulatory approval. AI is redefining this paradigm by accelerating the identification of new anticancer compounds, repurposing existing drugs, and improving patient access to experimental therapies.
In accelerated drug development, AI-powered algorithms can integrate chemical, structural, and biological datasets to identify novel drug candidates within months rather than years. Deep generative models, such as variational autoencoders and generative adversarial networks, can design molecules with optimized pharmacokinetic and pharmacodynamic properties [10, 20]. NLP applied to biomedical literature and patent databases can uncover overlooked targets or novel mechanisms of action [21]. Notably, AI-driven platforms have already advanced small molecules from in silico design to phase I clinical trials in under 18 months, demonstrating unprecedented speed [22]. AI also facilitates drug repurposing by mining transcriptomic and phenotypic data to match existing compounds with new oncology indications, reducing development costs and safety risks [23].
In clinical trial matching, AI tools can integrate tumor genomic profiles, EHR data, and trial eligibility criteria to recommend studies for which a patient may qualify [24, 25]. These systems can parse unstructured clinical notes, normalize genetic nomenclature, and dynamically update trial matches as patient data evolve. This capability is particularly valuable for rare cancers or those with uncommon driver mutations, where trial opportunities may otherwise be missed. AI-enabled trial matching not only accelerates patient enrollment but also increases access to cutting-edge targeted therapies and immunotherapies [26].
By compressing development timelines and expanding patient access to novel agents, AI has the potential to bring effective cancer treatments to the clinic faster and more equitably, ultimately improving survival and quality of life.
| Optimizing Radiotherapy and Surgery | ▴Top |
Radiotherapy and surgery remain central pillars of cancer treatment, and AI is transforming both modalities by improving precision, efficiency, and safety.
In radiotherapy planning, AI-driven auto-contouring systems can delineate tumors and organs at risk (OARs) from CT and MRI scans with accuracy comparable to or exceeding that of expert clinicians [27, 28]. These tools reduce inter-observer variability, shorten planning time, and enable highly conformal dose distributions that minimize irradiation of healthy tissue [29]. DL models can also predict normal tissue complication probabilities and tumor control probabilities, allowing individualized dose optimization [30]. By integrating imaging, genomic, and treatment response data, adaptive radiotherapy systems can update plans in real time as tumor morphology changes during treatment, further enhancing efficacy and sparing normal structures [31].
In surgical oncology, AI-enhanced image guidance is improving tumor localization and resection accuracy. Intraoperative augmented reality (AR) systems, powered by AI-based segmentation and registration algorithms, can overlay preoperative imaging onto the surgical field to help surgeons navigate complex anatomy [32]. Real-time tissue classification using hyperspectral imaging and DL can distinguish malignant from healthy tissue intraoperatively, supporting maximal tumor removal while preserving functionally important structures [33]. AI-assisted robotics further enhance precision in minimally invasive procedures, reducing operative times, complication rates, and recovery periods [34].
By integrating AI into radiotherapy and surgery, clinicians can deliver more targeted treatments with fewer side effects, optimize functional preservation, and improve long-term outcomes. These advancements align with the broader goal of precision oncology—delivering the right treatment, in the right dose, at the right location, for the right patient.
| Continuous Monitoring and Early Recurrence Detection | ▴Top |
AI has revolutionized post-treatment cancer management by enabling continuous monitoring and proactive detection of recurrence, significantly improving patient outcomes. Wearable devices and remote patient monitoring platforms generate real-time physiological data—including heart rate variability, activity levels, and sleep patterns—that AI algorithms analyze to detect deviations from a patient’s baseline indicative of treatment toxicity, infection, or early disease progression [35, 36]. For example, AI-enhanced wearable biosensors have been used to identify subclinical arrhythmias in patients receiving cardiotoxic chemotherapy, prompting timely intervention [37].
In recurrence surveillance, AI systems can detect minimal residual disease and subtle disease progression well before clinical symptoms manifest. Integration of serial imaging data (CT, MRI, PET) with laboratory results (e.g., tumor markers, ctDNA) allows ML models to identify high-risk patterns of recurrence with greater sensitivity than conventional follow-up protocols [38, 39]. AI-enabled ctDNA analysis can track clonal evolution and quantify tumor burden dynamics in real time, providing a non-invasive means to detect molecular relapse months earlier than radiographic detection [40].
Furthermore, predictive models incorporating longitudinal EHR data, treatment history, and multi-omics profiles can stratify patients by recurrence risk, enabling personalized follow-up schedules [41]. This targeted surveillance reduces unnecessary testing for low-risk patients while ensuring high-risk individuals to receive timely diagnostic evaluations.
By facilitating early detection of recurrence, AI-powered monitoring systems enable earlier therapeutic intervention, which is associated with improved survival and quality of life in multiple cancer types [42]. As these tools become more integrated into oncology care pathways, they have the potential to transform survivorship management from reactive to anticipatory, ultimately shifting the paradigm toward proactive cancer care.
| Enhanced Supportive and Palliative Care | ▴Top |
AI is increasingly being integrated into supportive and palliative care for cancer patients, aiming to improve quality of life, reduce symptom burden, and facilitate patient-clinician communication. One key application is symptom prediction and management. ML models trained on EHRs and patient-reported outcomes can predict the onset and severity of treatment-related adverse effects—such as chemotherapy-induced nausea, neuropathy, or mucositis—before they become debilitating [43, 44]. These systems can generate personalized recommendations, including dietary adjustments, physical therapy, and tailored pharmacologic interventions, thereby enabling proactive care and reducing unplanned hospital visits [45].
AI is also being used to optimize pain control, with algorithms capable of dynamically adjusting opioid dosing regimens based on patient-specific pharmacogenomic data and longitudinal pain assessments [46]. Similarly, AI can aid in early identification of patients at high risk for cachexia, enabling timely nutritional interventions [47].
Another promising area is mental health and communication support. Conversational AI systems and chatbots, trained on oncology-specific datasets, can provide emotional support, deliver evidence-based information, and guide patients through complex treatment decisions [48, 49]. These tools can reduce anxiety, enhance patient empowerment, and improve adherence to care plans, particularly for those with limited access to in-person counseling. Furthermore, AI-driven natural language processing can summarize and clarify clinical notes for patients, ensuring better comprehension of their diagnosis, treatment, and follow-up care [50].
By extending beyond disease-focused treatment to address holistic needs, AI-enabled supportive and palliative care has the potential to significantly improve both survival-adjusted quality of life and patient satisfaction. As these tools are integrated into routine oncology practice, careful evaluation of their clinical effectiveness, ethical considerations, and equitable accessibility will be essential.
| Health Equity and Access | ▴Top |
AI holds significant promise in reducing disparities in cancer care by improving access to timely diagnosis and personalized treatment for underserved populations. Tele-oncology platforms, powered by AI-driven decision support, enable remote consultations, image analysis, and treatment planning, allowing patients in rural or low-resource settings to receive specialized oncology expertise without the need for extensive travel [51, 52]. This is particularly valuable in regions with a shortage of oncologists, where delays in diagnosis and treatment initiation contribute to poorer outcomes [53].
NLP-based systems can translate complex medical terminology into clear, culturally sensitive language, bridging communication gaps for patients with low health literacy or those who speak minority languages [54]. Such tools can also generate patient-specific educational materials, enhancing comprehension of treatment plans and empowering individuals to participate actively in decision-making [55].
Moreover, AI algorithms trained on diverse datasets can help mitigate biases in clinical decision-making, ensuring that treatment recommendations are not disproportionately skewed toward populations historically overrepresented in biomedical research [56]. Integration of AI into community health programs can facilitate population-level cancer risk stratification, enabling earlier interventions and screening outreach in high-risk groups [57].
Importantly, equitable deployment of AI in oncology requires addressing infrastructure barriers, including internet access, device availability, and local capacity for implementation [58]. Partnerships between technology developers, healthcare systems, and public health agencies are essential to ensure that AI-enabled tools are validated across diverse demographic and socioeconomic groups before widespread adoption [59].
If implemented with attention to inclusivity and fairness, AI-enabled health equity strategies have the potential to significantly narrow the cancer survival gap between well-resourced and underserved populations, supporting global efforts toward universal cancer care [60].
In conclusion, AI is reshaping cancer care by enhancing precision, efficiency, and accessibility across the entire patient journey—from prevention and diagnosis to treatment and survivorship. AI enables earlier cancer detection through advanced imaging interpretation, liquid biopsy analysis, and pathology automation, improving the chances of successful intervention. By integrating genomic, clinical, and imaging data, AI supports personalized treatment planning, predicting therapy responses, and facilitating access to targeted drugs and relevant clinical trials. In radiotherapy and surgery, AI-driven planning and navigation tools increase accuracy, minimize harm to healthy tissues, and optimize recovery. Continuous monitoring systems powered by AI can detect complications or disease recurrence earlier than conventional follow-up methods, while supportive care tools help manage symptoms, maintain quality of life, and address psychological needs. Importantly, AI can bridge gaps in health equity through tele-oncology, language translation, and accessible health information, extending specialized cancer expertise to underserved populations. Although challenges remain—such as ensuring data privacy, avoiding bias, and validating models in diverse populations—the potential of AI to transform cancer care is profound. By integrating AI into clinical practice responsibly, healthcare systems can deliver more timely, precise, and patient-centered care, ultimately improving outcomes and quality of life for cancer patients worldwide.
Acknowledgments
We would like to express our sincere gratitude to Dr. Licun Wu for inviting me to contribute my manuscript to this innovative journal. His overarching vision and encouragement were instrumental in the development and completion of this article.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest to disclose.
Author Contributions
Nan Wu conceived the research idea and designed the study, wrote and finalized the manuscript.
Data Availability
The author declares that data supporting the findings of this study are available within the article.
| References | ▴Top |
- https://chatgpt.com/.
- https://chat.deepseek.com/.
- https://grok.com/.
- https://www.perplexity.ai/.
- McKinney SM, Sieniek M, Godbole V, Godwin J, Antropova N, Ashrafian H, Back T, et al. International evaluation of an AI system for breast cancer screening. Nature. 2020;577(7788):89-94.
doi pubmed - Ardila D, Kiraly AP, Bharadwaj S, Choi B, Reicher JJ, Peng L, Tse D, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25(6):954-961.
doi pubmed - Campanella G, Hanna MG, Geneslaw L, Miraflor A, Werneck Krauss Silva V, Busam KJ, Brogi E, et al. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat Med. 2019;25(8):1301-1309.
doi pubmed - Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8-17.
doi pubmed - Bibault JE, et al. Deep learning and radiomics predict survival in non-small cell lung cancer patients treated with radiotherapy. Radiother Oncol. 2016;120:1-6.
- Zhavoronkov A, Ivanenkov YA, Aliper A, Veselov MS, Aladinskiy VA, Aladinskaya AV, Terentiev VA, et al. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat Biotechnol. 2019;37(9):1038-1040.
doi pubmed - Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, Parkinson C, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497(7447):108-112.
doi pubmed - Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, Wang Y, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243.
doi pubmed - Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyo D, Moreira AL, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567.
doi pubmed - Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, Jensen SO, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570(7761):385-389.
doi pubmed - Ching T, Himmelstein DS, Beaulieu-Jones BK, Kalinin AA, Do BT, Way GP, Ferrero E, et al. Opportunities and obstacles for deep learning in biology and medicine. J R Soc Interface. 2018;15(141):20170387.
doi pubmed - Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, Colaprico A, et al. Comprehensive characterization of cancer driver genes and mutations. Cell. 2018;173(2):371-385.e318.
doi pubmed - Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, Cui C, et al. A guide to deep learning in healthcare. Nat Med. 2019;25(1):24-29.
doi pubmed - Trebeschi S, Drago SG, Birkbak NJ, Kurilova I, Calin AM, Delli Pizzi A, Lalezari F, et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann Oncol. 2019;30(6):998-1004.
doi pubmed - Zhang J, Fujimoto J, Zhang J, Wedge DC, Song X, Zhang J, Seth S, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346(6206):256-259.
doi pubmed - Sanchez-Lengeling B, Aspuru-Guzik A. Inverse molecular design using machine learning: Generative models for matter engineering. Science. 2018;361(6400):360-365.
doi pubmed - Wang LL, Lo K, Chandrasekhar Y, Reas R, Yang J, Burdick D, Eide D, et al. CORD-19: the COVID-19 open research dataset. ArXiv. 2020.
pubmed - Stokes JM, Yang K, Swanson K, Jin W, Cubillos-Ruiz A, Donghia NM, MacNair CR, et al. A deep learning approach to antibiotic discovery. Cell. 2020;180(4):688-702.e613.
doi pubmed - Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41-58.
doi pubmed - Shivade C, Raghavan P, Fosler-Lussier E, Embi PJ, Elhadad N, Johnson SB, Lai AM. A review of approaches to identifying patient phenotype cohorts using electronic health records. J Am Med Inform Assoc. 2014;21(2):221-230.
doi pubmed - Wu E, et al. Deep learning for pharmacogenomics and personalized medicine: From data to predictions and recommendations. Nat. Commun. 2021;12:1-13.
- McGary CS, et al. Patient matching algorithms for clinical trial recruitment: a review. JCO Clin. Cancer Inform. 2021;5:1001-1010.
- Men K, Chen X, Zhang Y, Zhang T, Dai J, Yi J, Li Y. Deep Deconvolutional Neural Network for Target Segmentation of Nasopharyngeal Cancer in Planning Computed Tomography Images. Front Oncol. 2017;7:315.
doi pubmed - Cardenas CE, Anderson BM, Aristophanous M, Yang J, Rhee DJ, McCarroll RE, Mohamed ASR, et al. Auto-delineation of oropharyngeal clinical target volumes using 3D convolutional neural networks. Phys Med Biol. 2018;63(21):215026.
doi pubmed - Vaassen F, Hazelaar C, Vaniqui A, Gooding M, van der Heyden B, Canters R, van Elmpt W. Evaluation of measures for assessing time-saving of automatic organ-at-risk segmentation in radiotherapy. Phys Imaging Radiat Oncol. 2020;13:1-6.
doi pubmed - Lim-Reinders S, et al. Technical innovations in radiation oncology: adaptive radiotherapy. Front Oncol. 2017;7:224.
- Bertholet J, Knopf A, Eiben B, McClelland J, Grimwood A, Harris E, Menten M, et al. Real-time intrafraction motion monitoring in external beam radiotherapy. Phys Med Biol. 2019;64(15):15TR01.
doi pubmed - Maier-Hein L, Vedula SS, Speidel S, Navab N, Kikinis R, Park A, Eisenmann M, et al. Surgical data science for next-generation interventions. Nat Biomed Eng. 2017;1(9):691-696.
doi pubmed - Jansen-Winkeln B, et al. Intraoperative use of hyperspectral imaging for tissue classification in surgery: a feasibility study. J Biophotonics. 2019;12:e201800455.
- Hashizume M, Tsugawa K. Robotic surgery and cancer: the present state, problems and future perspectives. Jpn J Clin Oncol. 2003;33:318-324.
- Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118.
doi pubmed - Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56.
doi pubmed - Luchini C, Pea A, Scarpa A. Artificial intelligence in oncology: current applications and future perspectives. Br J Cancer. 2022;126(1):4-9.
doi pubmed - Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts H. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18(8):500-510.
doi pubmed - Bera K, Schalper KA, Rimm DL, Velcheti V, Madabhushi A. Artificial intelligence in digital pathology - new tools for diagnosis and precision oncology. Nat Rev Clin Oncol. 2019;16(11):703-715.
doi pubmed - Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, Pacey S, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
doi pubmed - Wang Q, et al. Artificial intelligence–based imaging analysis for predicting prognosis in patients with colorectal cancer. JAMA Oncology. 2022;8:1300-1308.
- Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, Silliman N, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra392.
doi pubmed - Wong A, et al. Advances in precision oncology for cancer treatment. JAMA Oncol. 2022;8:585-592.
- Sklar MC, et al. Strategies for improving cancer care delivery. Lancet Oncol. 2023;24:e450-e459.
- Abernethy AP, et al. Enhancing oncology practice through data integration. J Oncol Pract. 2020;16:e584-e593.
- Liang Y, et al. Pain management in cancer patients. Pain. 2022;163:1872-1884.
- Morita T, et al. Palliative care approaches in advanced cancer. Palliat Med. 2022;36:835-844.
- Field M, Hardcastle N, Jameson M, Aherne N, Holloway L. Machine learning applications in radiation oncology. Phys Imaging Radiat Oncol. 2021;19:13-24.
doi pubmed - Chen M, et al. Psychological interventions for cancer patients. Psychooncology. 2022;31:1192-1200.
- Cai CJ, et al. Human-centered design in digital health tools. NPJ Digit Med. 2019;2:47.
- Dorsey ER, Topol EJ. Telemedicine in the era of COVID-19. Nat Med. 2020;26:463-464.
- Ohannessian R, et al. Global telemedicine implementation and integration within health systems. J Telemed Telecare. 2020;26:263-269.
- Anderson BO, et al. Breast cancer management in low-resource settings. Lancet Oncol. 2020;21:e375-e386.
- Reddy S, Fox J, Purohit MP. Artificial intelligence-enabled healthcare delivery. J R Soc Med. 2019;112(1):22-28.
doi pubmed - Jimenez G, et al. Digital health interventions for chronic disease management. BMJ Open. 2020;10:e035727.
- Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453.
doi pubmed - Duffy SW, et al. Breast cancer screening: Advances and challenges. CA Cancer J Clin. 2021;71:50-74.
- Scott RE, Mars M. Telehealth in the developing world: Current status and future prospects. Telemed J E-Health. 2015;21:323-332.
- Yu KH, et al. Artificial intelligence in medical imaging. Nat Biomed Eng. 2018;2:719-731.
- Wild CP, et al. Cancer prevention in the 21st century. Lancet. 2020;395:849-860.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
AI in Clinical Medicine is published by Elmer Press Inc.