| AI in Clinical Medicine, ISSN 0000-0000 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, AI Clin Med and Elmer Press Inc |
| Journal website https://aicm.elmerpub.com |
Review
Volume 1, 2025, e2
Historical Breakthroughs in Oncology: An AI-Assisted Perspective
Licun Wua, b, Chengke Zhangc, Tianhui Chend, e, g, Xiaogang Zhaoc, f, g
aLatner Thoracic Surgery Research Laboratories, Division of Thoracic Surgery, Toronto General Hospital, Toronto General Hospital Research Institute, University Health Network, University of Toronto, Toronto, ON M5G 1L7, Canada
bPrincess Margaret Cancer Centre, University Health Network, Toronto, ON M5G 1L7, Canada
cKey Laboratory of Thoracic Cancer, Cheeloo College of Medicine, Shandong University, Jinan 250033, Shandong Province, China
dDepartment of Cancer Prevention, Zhejiang Cancer Hospital, Hangzhou, China
eHangzhou Institute of Medicine (HIM), Chinese Academy of Sciences, Hangzhou, China
fDepartment of Thoracic Surgery, The Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan 250033, Shandong Province, China
gCorresponding Author: Tianhui Chen, Department of Cancer Prevention, Zhejiang Cancer Hospital, Hangzhou, China; Xiaogang Zhao, Key Laboratory of Thoracic Cancer, Cheeloo College of Medicine, Shandong University, Jinan 250033, Shandong Province, China
Manuscript submitted April 12, 2025, accepted April 17, 2025, published online April 25, 2025
Short title: Historical Breakthroughs in Oncology
doi: https://doi.org/10.14740/aicm2
- Abstract
- Introduction
- Ancient Understanding of Cancer (Pre-19th Century)
- The Birth of Modern Oncology (19th Century)
- Radiation Therapy (Late 19th - Early 20th Century)
- Chemotherapy (Mid-20th Century)
- Hormonal Therapy (20th Century)
- Advances in Surgical Oncology: Minimally Invasive Techniques (20th Century)
- Radiation Therapy Innovations (20th - 21st Century)
- Targeted Therapy: Precision Medicine (Late 20th - 21st Century)
- Immunotherapy: A New Era in Cancer Treatment (21st Century)
- Genomic Revolution: Transforming Cancer Diagnosis and Treatment (21st Century)
- Liquid Biopsy and Early Detection (21st Century)
- AI in Oncology: Transforming Cancer Care (21st Century)
- Future Direction
- Conclusion
- References
| Abstract | ▴Top |
The field of oncology has evolved dramatically over centuries, transitioning from ancient theories to modern, technology-driven treatments. This manuscript chronicles key milestones in cancer research and therapy, highlighting pivotal discoveries that have shaped the discipline. Early understandings of cancer, rooted in Hippocrates’ humoral theory and Galen’s black bile hypothesis, dominated until the 19th century, when Rudolf Virchow’s cellular pathology and advancements in microscopy revolutionized cancer science. The late 19th and early 20th centuries introduced radiation therapy, following Wilhelm Roentgen’s discovery of X-rays and Marie Curie’s work with radium, laying the foundation for targeted tumor treatments. The mid-20th century saw the rise of chemotherapy, beginning with nitrogen mustard and antimetabolites like methotrexate, while hormonal therapies emerged for breast and prostate cancers. Surgical innovations, including minimally invasive techniques and sentinel lymph node biopsies, reduced invasiveness and improved recovery. The 21st century ushered in precision medicine, with targeted therapies such as imatinib (chronic myeloid leukemia (CML)) and trastuzumab (human epidermal growth factor receptor 2-positive (HER2+) breast cancer), alongside breakthroughs in immunotherapy (checkpoint inhibitors, chimeric antigen receptor (CAR)-T cells) and genomic medicine (clustered regularly interspaced short palindromic repeats (CRISPR), liquid biopsies). Artificial intelligence (AI) now enhances diagnostics, drug discovery, and personalized treatment planning. Future directions emphasize early detection, AI-driven precision oncology, and global collaboration to transform cancer into a manageable condition. This historical perspective underscores the remarkable progress in oncology, driven by scientific innovation, and envisions a future where cancer care is increasingly effective, personalized, and accessible.
Keywords: Oncology; Cancer therapy; Precision medicine; Immunotherapy; Chemotherapy; Radiation therapy; Artificial intelligence; Genomic medicine
| Introduction | ▴Top |
The field of oncology has seen remarkable advancements over the centuries, driven by scientific discoveries, technological innovations, and a deeper understanding of cancer biology [1]. Below is an artificial intelligence (AI)-assisted insight into the historic milestones in oncology, highlighting key developments that have shaped cancer research and treatment [2, 3] (Table 1, Fig. 1).
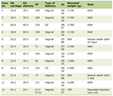 Click to view | Table 1. Key Milestones in Oncology: From Ancient Theories to AI |
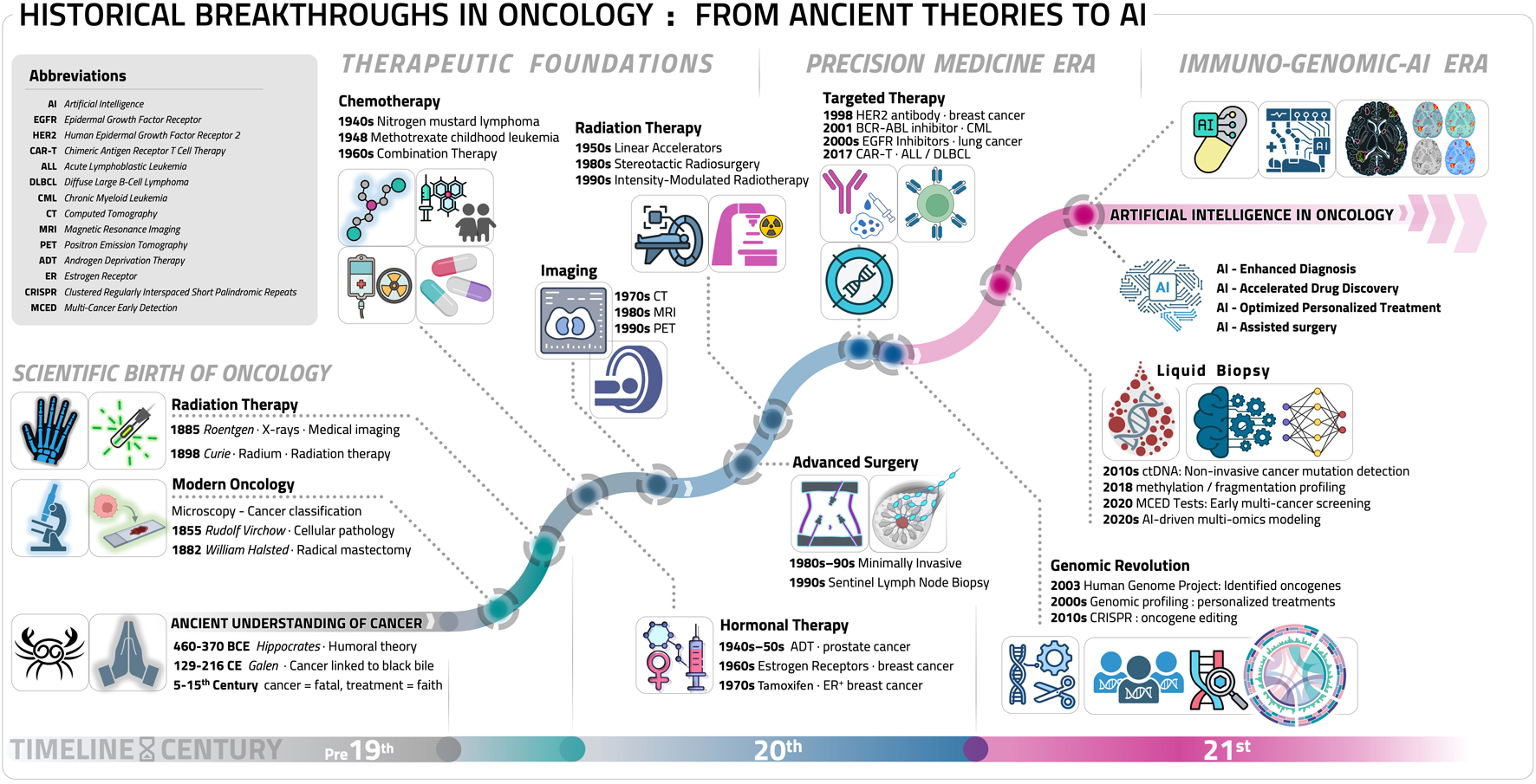 Click for large image | Figure 1. Key milestones in oncology: a chronological artificial intelligence (AI) perspective. This timeline diagram illustrates the major milestone breakthroughs in oncology, spanning from ancient theories to cutting-edge innovations: ancient understanding of cancer (pre-19th century), the birth of modern oncology (19th century), radiation therapy (late 19th - early 20th century), chemotherapy (mid-20th century), hormonal therapy (20th century), advances in surgical oncology: minimally invasive techniques (20th century), radiation therapy innovations (20th - 21st century), targeted therapy: precision medicine (late 20th - 21st century), immunotherapy: a new era in cancer treatment (21st century), genomic revolution: transforming cancer diagnosis and treatment (21st century), liquid biopsy and early detection (21st century), AI in oncology: transforming cancer care (21st century). Some elements in this figure, including specific icons and biological illustrations, were created using BioRender (BioRender.com). |
| Ancient Understanding of Cancer (Pre-19th Century) | ▴Top |
Hippocrates (460 - 370 BCE): Coined the term “karkinos” (Greek for crab) to describe tumors, likening their appearance to crabs. He introduced the humoral theory, suggesting cancer was caused by an imbalance of bodily fluids.
Galen (129 - 216 CE): Expanded on Hippocrates’ ideas, attributing cancer to an excess of black bile.
Middle Ages (5th - 15th century): Cancer was often considered incurable, and surgical interventions were rare and crude.
During the Middle Ages, medical advancements stagnated, and cancer was largely regarded as a fatal and untreatable affliction. Surgical interventions were rare due to limited anatomical knowledge, rudimentary surgical techniques, and the risk of infection. Instead, treatments were largely palliative or based on religious and mystical beliefs rather than scientific understanding. The lack of effective interventions and the dominance of humoral theory prevented significant progress in cancer treatment until the advent of modern medical science in the 19th century.
The historical understanding of cancer, from Hippocrates to the pre-modern era, was deeply rooted in humoral theory and limited medical knowledge. Hippocrates (460 - 370 BCE), often called the “Father of Medicine,” first described tumors using the Greek term karkinos (crab), inspired by their appearance [4]. He theorized that cancer resulted from an excess of black bile, a central idea in humoral medicine [5]. Galen (129 - 216 CE), a prominent Roman physician, reinforced this belief, asserting that black bile buildup made cancer largely incurable [6]. His ideas dominated medical thought for centuries.
During the Middle Ages, medical progress stagnated, and cancer was viewed as untreatable. Treatments were often palliative, relying on religious or mystical beliefs rather than scientific understanding [7]. Surgical removal of tumors was rare due to limited anatomical knowledge, primitive techniques, and infection risks [8]. The dominance of humoral theory persisted until the 19th century when advances in pathology and cellular biology led to scientific approaches in cancer treatment. The shift from ancient theories to modern oncology paved the way for groundbreaking discoveries in cancer research.
Overall, early conceptions of cancer were rooted in theoretical frameworks rather than empirical evidence, and it was not until later centuries that scientific advancements enabled a deeper understanding of its causes and potential treatments.
| The Birth of Modern Oncology (19th Century) | ▴Top |
Rudolf Virchow (1821 - 1902): Pioneered cellular pathology, identifying that cancer originates from cells, not humors.
Development of Microscopy (19th century): Enabled the study of cancer cells and tissues, leading to the classification of different cancer types.
First Mastectomy (1882): William Halsted performed the first radical mastectomy for breast cancer, emphasizing surgical removal of tumors.
The 19th century marked the birth of modern oncology, driven by groundbreaking discoveries in cellular pathology, microscopy, and surgical techniques. Rudolf Virchow (1821 - 1902), a pioneering German pathologist, revolutionized cancer research by establishing that cancer originates from cells rather than an imbalance of bodily humors [9]. His work in cellular pathology refuted humoral theory, shifting cancer research from a theoretical to a scientific framework [10].
The advancement of microscopy played a crucial role in this transformation. With improved magnification and staining techniques, scientists could examine cancerous tissues at the cellular level, leading to the classification of various cancer types [11]. This breakthrough allowed for more precise diagnoses and a deeper understanding of tumor behavior.
Surgical oncology also progressed significantly. In 1882, William Halsted introduced the radical mastectomy for breast cancer, emphasizing complete tumor removal to prevent recurrence [12]. Though aggressive, his approach laid the foundation for modern surgical techniques and demonstrated the importance of localized cancer treatment [13].
Furthermore, anesthesia and antiseptic techniques pioneered by Joseph Lister improved the safety and efficacy of cancer surgeries, reducing mortality rates [14]. These developments, along with the emergence of histopathology, transformed cancer treatment, moving beyond speculative theories toward evidence-based medical practice.
The scientific breakthroughs of the 19th century laid the foundation for modern oncology. The transition from humoral explanations to cellular pathology set the stage for future cancer research, leading to innovations in diagnosis, treatment, and ultimately, improved patient outcomes.
| Radiation Therapy (Late 19th - Early 20th Century) | ▴Top |
Discovery of X-rays (1895): Wilhelm Roentgen’s discovery led to the use of radiation for cancer treatment.
Radium Therapy (Early 1900s): Marie Curie’s discovery of radium paved the way for radiation therapy, particularly for cervical and skin cancers.
The late 19th and early 20th centuries marked a revolutionary shift in cancer treatment with the introduction of radiation therapy. In 1895, Wilhelm Roentgen discovered X-rays, allowing physicians to visualize internal structures without invasive surgery [15]. This breakthrough led researchers to explore the therapeutic potential of X-rays in treating tumors, marking the beginning of radiation-based oncology [16].
Building on Roentgen’s discovery, Marie Curie and Pierre Curie isolated radium in the early 1900s, demonstrating its potent radioactive properties [17]. Radium’s ability to emit continuous, powerful radiation made it an effective tool for cancer treatment, particularly for cervical and skin cancers [18]. Physicians soon developed radium therapy, using radium implants to deliver localized radiation directly to tumors, reducing the need for radical surgery [19].
Despite its effectiveness, early radiation therapy had significant limitations. Patients often suffered from severe burns and radiation sickness due to uncontrolled exposure and a lack of precision in dosage [20]. However, over time, advancements in radiation delivery methods, including fractionated dosing and improved shielding techniques, enhanced both safety and therapeutic outcomes. These developments transformed radiation therapy into a cornerstone of modern oncology, enabling more targeted and effective cancer treatments.
The discoveries of X-rays and radium laid the foundation for contemporary radiation oncology, influencing innovations such as intensity-modulated radiation therapy (IMRT) and proton therapy. These advancements continue to shape cancer treatment, offering patients safer and more precise therapeutic options.
| Chemotherapy (Mid-20th Century) | ▴Top |
Nitrogen Mustard (1940s): Initially developed as a chemical weapon, it was repurposed as the first chemotherapy drug for lymphoma.
Discovery of Antimetabolites (1948): Methotrexate, a folate antagonist, became the first drug to induce remission in childhood leukemia.
Combination Chemotherapy (1960s): The use of multiple drugs (e.g., for Hodgkin’s lymphoma) significantly improved outcomes.
The mid-20th century marked a major breakthrough in oncology with the development of chemotherapy. During the 1940s, researchers discovered that nitrogen mustard, originally developed as a chemical weapon, could selectively kill rapidly dividing cells [21]. This led to its repurposing as the first chemotherapy drug, successfully treating lymphoma and paving the way for further drug-based cancer therapies [22].
In 1948, the discovery of antimetabolites further revolutionized chemotherapy. Sidney Farber demonstrated that methotrexate, a folate antagonist, could induce remission in childhood leukemia, proving that chemical agents could effectively target cancer cells [23]. This milestone spurred the development of additional drugs designed to interfere with cancer cell growth and replication [24].
By the 1960s, the introduction of combination chemotherapy transformed cancer treatment. Physicians, including Vincent DeVita, found that using multiple drugs with different mechanisms of action significantly improved survival rates, particularly in Hodgkin’s lymphoma [25]. This approach not only increased treatment effectiveness but also helped prevent drug resistance, a major challenge in cancer therapy [26].
Chemotherapy’s evolution from a wartime discovery to a powerful medical tool revolutionized oncology. Over time, refinements in drug development, targeted therapies, and better administration techniques have enhanced efficacy while reducing toxicity. These advancements have shaped modern cancer care, turning chemotherapy into a cornerstone of cancer treatment.
| Hormonal Therapy (20th Century) | ▴Top |
Discovery of Hormone Receptors (1960s): Identification of estrogen receptors in breast cancer led to the development of tamoxifen, a groundbreaking hormonal therapy.
Androgen Deprivation Therapy (1940s - 1950s): Used for prostate cancer, marking the beginning of targeted hormonal treatments.
The 20th century brought significant advancements in cancer treatment with the introduction of hormonal therapy, particularly for hormone-dependent cancers such as breast and prostate cancer. Unlike traditional treatments like surgery or chemotherapy, hormonal therapy targeted the molecular mechanisms driving cancer growth.
In the 1940s and 1950s, researchers discovered that prostate cancer progression was influenced by androgens [27]. This led to the development of androgen deprivation therapy (ADT), which either reduced androgen levels or blocked their effects, slowing tumor growth [28]. ADT became a cornerstone of prostate cancer treatment and remains a standard approach today [29].
Further breakthroughs occurred in the 1960s with the discovery of estrogen receptors in breast cancer cells [30]. Scientists found that some breast cancers depended on estrogen for growth, leading to the development of tamoxifen, a selective estrogen receptor modulator (SERM) [31]. Tamoxifen revolutionized breast cancer treatment by effectively blocking estrogen’s effects, significantly improving survival rates in estrogen receptor-positive patients [32].
These discoveries transformed oncology by introducing targeted therapies that addressed cancer at a molecular level. Today, hormonal therapies continue to evolve, with newer agents like aromatase inhibitors and androgen receptor blockers offering more precise and effective treatments for hormone-driven cancers.
| Advances in Surgical Oncology: Minimally Invasive Techniques (20th Century) | ▴Top |
Minimally Invasive Surgery (1980s - 1990s): Laparoscopic and robotic techniques reduced recovery times and improved precision.
Sentinel Lymph Node Biopsy (1990s): Reduced the need for extensive lymph node removal in breast cancer and melanoma.
The 20th century witnessed transformative advancements in surgical oncology, improving precision, reducing invasiveness, and enhancing patient outcomes. These innovations redefined cancer surgery, making procedures safer and more effective.
In the 1980s and 1990s, the advent of minimally invasive surgery revolutionized cancer treatment. Laparoscopic techniques, introduced in the late 1980s, allowed surgeons to perform operations using small incisions and specialized instruments, leading to reduced trauma, shorter recovery times, and fewer complications [33]. By the late 1990s, robotic-assisted surgery, pioneered by the da Vinci Surgical System, further enhanced precision, offering improved visualization and control, particularly in prostate and gynecologic cancers [34]. These innovations significantly improved patient recovery while maintaining surgical effectiveness.
Another major breakthrough in the 1990s was the development of sentinel lymph node biopsy (SLNB), which transformed the surgical management of breast cancer and melanoma. Traditionally, extensive lymph node removal was the standard, often resulting in complications such as lymphedema [35]. SLNB, introduced by Donald Morton and colleagues, allowed surgeons to identify and remove only the sentinel lymph nodes, the first to which cancer spreads, minimizing unnecessary surgery and reducing complications [36].
These advancements marked a shift toward more targeted, patient-friendly surgical approaches. Minimally invasive techniques and SLNB continue to be refined, contributing to improved cancer treatment with fewer complications and better quality of life for patients. The evolution of surgical oncology underscores the growing emphasis on precision and personalized treatment strategies.
| Radiation Therapy Innovations (20th - 21st Century) | ▴Top |
Linear Accelerators (1950s): Enabled more precise and controlled delivery of radiation.
Stereotactic Radiosurgery (1980s): Techniques like Gamma Knife and CyberKnife allowed for highly targeted radiation, sparing healthy tissue.
Proton Therapy (21st century): Uses protons instead of X-rays, reducing damage to surrounding tissues.
Radiation therapy has undergone remarkable advancements from the mid-20th century to the present, enhancing precision, reducing side effects, and improving patient outcomes. These innovations have transformed cancer treatment by minimizing damage to healthy tissue while maximizing tumor control.
In the 1950s, the introduction of linear accelerators revolutionized radiation therapy. Unlike earlier cobalt-60 machines, which exposed healthy tissues to excessive radiation, linear accelerators enabled the precise delivery of high-energy X-ray beams, significantly improving treatment effectiveness [37]. This development laid the foundation for modern external beam radiation therapy.
The 1980s saw further innovation with stereotactic radiosurgery (SRS), which provided highly focused radiation for brain and spinal tumors. Techniques such as Gamma Knife and CyberKnife allowed for precise targeting, delivering high-dose radiation with extreme accuracy while sparing surrounding healthy tissue [38, 39]. These non-invasive methods enabled treatment of previously inoperable tumors with fewer complications.
In the 21st century, proton therapy emerged as a cutting-edge advancement. Unlike traditional X-ray radiation, proton therapy uses charged protons that precisely target tumors while minimizing exposure to adjacent healthy tissues, making it especially beneficial for pediatric cancers and tumors near critical structures [40]. Its superior dose distribution has led to improved outcomes with fewer long-term side effects.
These advancements have transformed radiation therapy into a safer and more effective cancer treatment. Ongoing research continues to refine these technologies, integrating AI and adaptive radiation therapy to further enhance precision and patient care.
| Targeted Therapy: Precision Medicine (Late 20th - 21st Century) | ▴Top |
Imatinib (Gleevec) (2001): A breakthrough for chronic myeloid leukemia (CML), targeting the BCR-ABL fusion protein.
Human epidermal growth factor receptor 2 (HER2)-Targeted Therapy (1998): Trastuzumab (Herceptin) revolutionized treatment for HER2-positive breast cancer.
Epidermal growth factor receptor (EGFR) Inhibitors (2000s): Drugs like gefitinib and erlotinib targeted EGFR mutations in lung cancer.
The late 20th and early 21st centuries ushered in a new era of cancer treatment with the development of targeted therapies, which focus on specific molecular abnormalities driving cancer growth. These innovations have revolutionized oncology by offering more effective and less toxic alternatives to traditional treatments.
A major breakthrough came in 2001 with the approval of imatinib (Gleevec), a targeted therapy for CML. Imatinib inhibits the BCR-ABL fusion protein, which is responsible for uncontrolled cell division in CML. This drug transformed CML from a fatal disease into a manageable condition with significantly improved survival rates [41].
Another milestone occurred in 1998 with the introduction of trastuzumab (Herceptin), the first HER2-targeted therapy for HER2-positive breast cancer. By blocking the HER2 receptor, which promotes aggressive tumor growth, trastuzumab dramatically improved patient outcomes and became a cornerstone of breast cancer treatment [42].
The 2000s saw further progress with the development of EGFR inhibitors such as gefitinib and erlotinib for lung cancer. These drugs selectively target EGFR mutations, which drive tumor growth in specific lung cancer subtypes, offering more effective and personalized treatment options compared to traditional chemotherapy [43].
Targeted therapies have revolutionized cancer treatment by providing personalized approaches that improve efficacy while reducing side effects. Ongoing research continues to expand their scope, with new drugs targeting different molecular pathways, offering hope for improved cancer outcomes in the future.
| Immunotherapy: A New Era in Cancer Treatment (21st Century) | ▴Top |
Checkpoint Inhibitors (2011): Drugs like ipilimumab (anti-CTLA-4) and pembrolizumab (anti-PD-1) unlocked the immune system’s ability to fight cancer.
Chimeric antigen receptor T-cell (CAR-T) Therapy (2017): Genetically engineered T cells were approved for certain blood cancers, marking a new era in personalized medicine.
Cancer Vaccines (2006): The HPV vaccine (2006) prevents cervical cancer, and mRNA vaccines are being explored for therapeutic use.
The 21st century has brought about transformative advancements in cancer treatment, with immunotherapy emerging as a powerful tool in harnessing the body’s immune system to fight cancer more effectively. This shift has revolutionized oncology by offering new options for patients, especially those with difficult-to-treat cancers.
A groundbreaking development occurred in 2011 with the approval of checkpoint inhibitors, such as ipilimumab (anti-cytotoxic T lymphocyte antigen-4 (CTLA-4)) and pembrolizumab (anti-programmed cell death-1 (PD-1)). These drugs work by blocking immune checkpoint proteins, such as CTLA-4 and PD-1, which cancer cells use to evade immune detection. By inhibiting these checkpoints, the immune system is able to recognize and attack tumors more effectively. This marked a significant shift toward immunotherapy as a mainstream treatment modality, particularly for melanoma and non-small cell lung cancer [44, 45].
In 2017, CAR-T therapy was approved for blood cancers such as leukemia and lymphoma. This innovative treatment involves genetically modifying a patient’s own T cells to recognize and destroy cancer cells. CAR-T therapy has demonstrated remarkable success in treating refractory blood cancers, offering a personalized approach to cancer therapy [46].
Additionally, cancer vaccines have made significant progress. The human papillomavirus (HPV) vaccine, approved in 2006, prevents cervical cancer by targeting the HPV, a leading cause of the disease. Furthermore, mRNA vaccines, initially developed for COVID-19, are being explored for cancer treatment. These vaccines offer promising avenues for personalized cancer vaccines by targeting tumor-specific antigens [47].
Immunotherapy continues to transform cancer treatment by offering novel and targeted therapeutic options, improving survival rates, and enhancing patient quality of life. Ongoing research holds great potential for further expanding its applications across various cancer types.
| Genomic Revolution: Transforming Cancer Diagnosis and Treatment (21st Century) | ▴Top |
Human Genome Project (2003): Enabled the identification of cancer-related genes and mutations.
Precision Medicine (2000s): Genomic profiling allows for tailored treatments based on individual tumor genetics.
Clustered regularly interspaced short palindromic repeats (CRISPR) and Gene Editing (2010s): Emerging technologies hold promise for correcting genetic mutations driving cancer.
The 21st century has been marked by a genomic revolution that has dramatically advanced cancer diagnosis and treatment, ushering in an era of precision medicine. This transformation began with the completion of the Human Genome Project in 2003, which provided a comprehensive map of the human genome. This breakthrough allowed scientists to identify cancer-related genes and mutations, offering critical insights into the genetic basis of cancer [48]. Understanding these genetic alterations has been pivotal in shaping new, more effective treatment strategies.
One of the most significant outcomes of genomic research is the rise of precision medicine in oncology. Through genomic profiling, clinicians can now analyze the genetic makeup of individual tumors and tailor treatments to the specific mutations driving a patient’s cancer. This personalized approach enhances the effectiveness of treatments while minimizing side effects, as therapies are designed to target only the tumor’s unique genetic characteristics [49].
Emerging technologies, such as CRISPR and gene editing, are poised to further revolutionize cancer treatment. These tools allow for precise editing of genetic material, potentially correcting mutations that cause cancer. Although still in the experimental phase, CRISPR holds immense promise for developing novel therapeutic strategies that could prevent or even cure genetic cancers [50].
Together, these genomic advancements are not only transforming cancer care today but also laying the groundwork for future breakthroughs that could reshape the landscape of cancer treatment.
| Liquid Biopsy and Early Detection (21st Century) | ▴Top |
Circulating Tumor DNA (ctDNA) (2010s): Non-invasive blood tests can detect cancer mutations and monitor treatment response.
Multi-Cancer Early Detection (MCED) Tests (2010s): Emerging technologies aim to detect multiple cancers at early stages through blood samples.
The 21st century has witnessed remarkable advancements in cancer detection, particularly with the development of liquid biopsy, a non-invasive method that is revolutionizing cancer diagnosis and monitoring. One of the key innovations in liquid biopsy is the use of ctDNA, which consists of small fragments of cancerous DNA released into the bloodstream. By analyzing ctDNA through blood tests, clinicians can detect cancer-related mutations, monitor treatment responses, and identify minimal residual disease. This approach provides a less invasive and more accessible alternative to traditional tissue biopsies, enabling frequent monitoring of cancer progression without the need for additional surgical procedures [51].
In addition to ctDNA, another exciting development is the emergence of MCED tests. These technologies aim to detect a variety of cancers at their earliest, most treatable stages by analyzing blood samples for cancer-related biomarkers. MCED tests have the potential to revolutionize cancer screening by identifying cancers that may otherwise go undiagnosed until more advanced stages, thus improving survival rates and treatment outcomes [52].
Together, liquid biopsy and MCED tests represent a significant leap forward in early cancer detection. These non-invasive, precise methods enable clinicians to diagnose and monitor cancers more effectively, ultimately leading to earlier interventions and better patient outcomes.
| AI in Oncology: Transforming Cancer Care (21st Century) | ▴Top |
AI for Diagnosis: Machine learning algorithms improve the accuracy of cancer detection in imaging and pathology.
Drug Discovery: AI accelerates the identification of new drug targets and therapies.
Personalized Treatment Plans: AI analyzes patient data to recommend optimal treatment strategies.
In the 21st century, AI has become a transformative force in oncology, revolutionizing many aspects of cancer care. One of the most significant contributions of AI has been in the realm of cancer diagnosis. Machine learning algorithms, which can analyze medical imaging and pathology slides, have enhanced the accuracy and speed of cancer detection. These algorithms are capable of detecting subtle patterns in imaging data that may be missed by human experts, allowing for earlier and more accurate diagnoses. AI’s ability to process vast amounts of data quickly and consistently has had a profound impact on fields such as radiology and histopathology, where it is used to detect tumors, classify cancer types, and even predict patient outcomes [53].
In drug discovery, AI is accelerating the identification of new drug targets and therapies. By analyzing large datasets of genetic, molecular, and clinical information, AI can predict which compounds are most likely to be effective for specific cancer types. This approach helps to reduce the time and cost associated with developing new drugs, enabling faster delivery of innovative treatments to patients [54].
AI is also central to the creation of personalized treatment plans. By analyzing a patient’s unique genetic, clinical, and treatment history, AI can recommend the most effective therapies tailored to their specific cancer profile. This personalized approach improves treatment outcomes by targeting the individual characteristics of each patient’s disease [55].
Overall, AI is reshaping oncology, improving diagnosis, accelerating drug discovery, and enabling personalized treatment strategies that enhance the effectiveness of cancer care.
| Future Direction | ▴Top |
The future direction of oncology is poised to be transformative, driven by rapid advancements in technology, deeper understanding of cancer biology, and the integration of innovative approaches. Based on the historical milestones and current trends, the future of oncology will focus on precision, prevention, and personalization. Advancements in AI, immunotherapy, genomics, and early detection are poised to transform oncology by enhancing efficacy, minimizing invasiveness, and improving accessibility [56-58]. Multidisciplinary and international collaboration will be critical to implementing these innovations and ultimately shifting cancer from a life-threatening disease to a manageable chronic condition.
| Conclusion | ▴Top |
The history of oncology is a testament to human ingenuity and perseverance. From ancient theories to cutting-edge technologies, each milestone has brought us closer to understanding and conquering cancer. The integration of genomics, immunotherapy, and AI promises a bright future direction of oncology, with a focus on precision, prevention, and personalization. Advances in AI, immunotherapy, genomics, and early detection will revolutionize cancer care, making it more effective, less invasive, and accessible, with global collaboration key to transforming cancer into a manageable condition.
Acknowledgments
The figure and table were created by Dr. Kui Chen, PhD, from the Translational Cancer Genomics Program at the Princess Margaret Cancer Centre and the Schwartz Reisman Liver Research Centre, Toronto General Hospital, University Health Network, Toronto, ON, Canada. We sincerely appreciate his generous contribution.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
Licun Wu: writing - original draft, validation, investigation, data curation, methodology, and conceptualization. Chengke Zhang: writing - review and editing. Tianhui Chen: writing - review and editing, supervision, methodology, investigation, and conceptualization. Xiaogang Zhao: writing - review and editing, supervision, methodology, investigation, and conceptualization.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- https://www.nobelprize.org/.
- https://chat.deepseek.com/.
- https://chatgpt.com/.
- National Cancer Institute. A Historical Perspective on Cancer. 2021.
- Porter R. The greatest benefit to mankind: a medical history of humanity. W.W. Norton & Company. 1997.
- Bynum W. The history of medicine: a very short introduction. Oxford University Press. 2008.
- Worboys M. The history of medicine and disease. Palgrave. 2016.
- Nuland S. The doctors: a history of medicine. Vintage. 2003.
- Virchow R. Cellular Pathology as Based Upon Physiological and Pathological Histology. 1858.
- Ackerknecht EH. Rudolf Virchow: doctor, statesman, anthropologist. University of Wisconsin Press. 1953.
- Porter R. The greatest benefit to mankind: a medical history of humanity. W.W. Norton & Company. 1997.
- Halsted WS. I. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894;20(5):497-555.
doi pubmed - Mukherjee S. The emperor of all maladies: a biography of cancer. Scribner. 2010.
- Nuland S. The doctors: a history of medicine. Vintage. 2003.
- Roentgen WC. Uber eine neue Art von Strahlen. Annalen der Physik. 1895;64(1):1-37.
- Kevles BH. Naked to the bone: medical imaging in the twentieth century. Rutgers University Press. 1997.
- Curie M. Recherches sur les substances radioactives. Gauthier-Villars. 1903.
- Pottier J. The legacy of marie curie in radiation oncology. Cancer Radiotherapie. 2001;5(4):357-363.
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Wolters Kluwer. 2018.
- Tubiana M. Radiation Oncology: A Century of Advances. Springer. 2005.
- Goodman LS, Gilman A. The Pharmacological Basis of Therapeutics. Macmillan. 1946.
- DeVita VT, Jr., Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643-8653.
doi pubmed - Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238(23):787-793.
doi pubmed - Mukherjee S. The emperor of all maladies: a biography of cancer. Scribner. 2010.
- Devita VT, Jr., Serpick AA, Carbone PP. Combination chemotherapy in the treatment of advanced Hodgkin's disease. Ann Intern Med. 1970;73(6):881-895.
doi pubmed - Chabner BA, Roberts TG, Jr. Timeline: chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5(1):65-72.
doi pubmed - Huggins C, Hodges CV. Studies on prostatic cancer: I. the effect of castration, estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research. 1941;1(4):293-297.
- Huggins C. Endocrine-induced regression of cancers. Cancer Res. 1967;27(11):1925-1930.
pubmed - Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, Blumenstein BA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321(7):419-424.
doi pubmed - Jensen EV, Jacobsen HI. Basic mechanism of estrogen action. Recent Progress in Hormone Research. 1962;18:387-414.
- Jordan VC. Effect of tamoxifen (ICI 46,474) on growth of DMBA-induced rat mammary carcinoma. European Journal of Cancer. 1976;12(4):419-424.
- Early Breast Cancer Trialists' Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319(26):1681-1692.
doi pubmed - Mouret P. How I developed laparoscopic cholecystectomy. Annales de Chirurgie. 1987;41(8):124-130.
- Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001;87(4):408-410.
doi pubmed - Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392-399.
doi pubmed - Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of sentinel lymph nodes in breast cancer using a gamma probe. Surgical Oncology. 1998;7(1):39-46.
- Kaplan HS. The evolution of radiation therapy. Cancer Research. 1966;26(6):1182-1191.
- Leksell L. The Leksell gamma knife. Journal of Neurosurgery. 1983;59(2):288-293.
- Adler JR, Jr., Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL. The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69(1-4 Pt 2):124-128.
doi pubmed - Suit H, DeLaney T, Goldberg S, Paganetti H, Clasie B, Gerweck L, Niemierko A, et al. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother Oncol. 2010;95(1):3-22.
doi pubmed - Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031-1037.
doi pubmed - Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-792.
doi pubmed - Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129-2139.
doi pubmed - Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-1546.
doi pubmed - Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
doi pubmed - Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448.
doi pubmed - Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261-279.
doi pubmed - Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860-921.
doi pubmed - Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793-795.
doi pubmed - Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096.
doi pubmed - Pantel K, Alix-Panabieres C. Liquid biopsy: the pathologist’s view. Journal of Pathology. 2019;248(1):3-7.
- Mandelker D, et al. Circulating tumor DNA testing in cancer care. Cancer Discovery. 2020;10(8):1157-1166.
- Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, Cui C, et al. A guide to deep learning in healthcare. Nat Med. 2019;25(1):24-29.
doi pubmed - Chen H, et al. Artificial intelligence in drug discovery: a review. Journal of Pharmaceutical Sciences. 2020;109(5):1675-1682.
- Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8-17.
doi pubmed - Zhang B, et al. Advancements in Cancer Genomics. Journal of Clinical Oncology. 2020;38(5):1-9.
- Wang Z, et al. Artificial intelligence in oncology: current trends and future directions. Cancer Research. 2021;81(12):2347-2358.
- Roberts C, et al. Immunotherapy: the next frontier in cancer treatment. Journal of Clinical Immunology. 2019;39(6):758-767.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
AI in Clinical Medicine is published by Elmer Press Inc.